Λίστα αντικειμένων
BACKGROUND
Evidence from a recent trial has shown that the antiinflammatory effects of colchicine reduce the risk of cardiovascular events in patients with recent myocardial infarction, but evidence of such a risk reduction in patients with chronic coronary
disease is limited.
METHODS
In a randomized, controlled, double-blind trial, we assigned patients with chronic coronary disease to receive 0.5 mg of colchicine once daily or matching placebo.
The primary end point was a composite of cardiovascular death, spontaneous (non-procedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization. The key secondary end point was a composite of cardiovascular death, spontaneous myocardial infarction, or ischemic stroke.
RESULTS
A total of 5522 patients underwent randomization; 2762 were assigned to the colchicine group and 2760 to the placebo group. The median duration of follow-up was 28.6 months. A primary end-point event occurred in 187 patients (6.8%) in the colchicine group and in 264 patients (9.6%) in the placebo group (incidence, 2.5 vs. 3.6 events per 100 person-years; hazard ratio, 0.69; 95% confidence interval [CI], 0.57 to 0.83; P<0.001). A key secondary end-point event occurred in 115 patients (4.2%) in the colchicine group and in 157 patients (5.7%) in the placebo group (incidence, 1.5 vs. 2.1 events per 100 person-years; hazard ratio, 0.72; 95% CI, 0.57 to 0.92; P=0.007). The incidence rates of spontaneous myocardial infarction or ischemia-driven coronary revascularization (composite end point), cardiovascular death or spontaneous myocardial infarction (composite end point), ischemia-driven coronary revascularization, and spontaneous myocardial infarction were also significantly lower with colchicine than with placebo. The incidence of death from noncardiovascular causes was higher in the colchicine group than in the placebo group (incidence, 0.7 vs. 0.5 events per 100 person-years; hazard ratio, 1.51; 95% CI, 0.99 to 2.31).
CONCLUSIONS
In a randomized trial involving patients with chronic coronary disease, the risk of cardiovascular events was significantly lower among those who received 0.5 mg of colchicine once daily than among those who received placebo. (Funded by the National Health Medical Research Council of Australia and others; LoDoCo2 Australian New Zealand Clinical Trials Registry number, ACTRN12614000093684.)
Despite lifestyle changes and risk factor reduction, patients with chronic coronary disease remain at high risk for
acute cardiovascular events.1-3 The central role of inflammation in the progression of coronary disease is well recognized.4,5 The possibility that antiinflammatory therapy may improve cardiovascular outcomes was first highlighted in the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) involving patients with a history of myocardial infarction and an elevated baseline level of C-reactive protein; the results showed that the risk of recurrent cardiovascular
events was lower among those who received canakinumab than among those who received placebo.6
However, in another trial, a clinical benefit with methotrexate was not observed in patients with chronic coronary disease.7
Colchicine is an antiinflammatory drug originally extracted from the autumn crocus (Colchicum autumnale) and was used by the ancient Greeks and Egyptians. In contrast to selective inhibition of interleukin-1β by canakinumab, colchicine
has broad cellular effects that include inhibition of tubulin polymerization and alteration of leukocyte responsiveness.8-10 In the Colchicine Cardiovascular Outcomes Trial (COLCOT) involving patients who had a myocardial infarction within
30 days before enrollment, the percentage of those who had the composite end point of cardiovascular death, resuscitated cardiac arrest, myocardial infarction, stroke, or urgent hospitalization for angina leading to coronary revascularization was
lower among those who received 0.5 mg of colchicine once daily than among those who received placebo.11
In an earlier trial of low-dose colchicine (LoDoCo) involving patients with chronic coronary disease, we found that the risk of acute cardiovascular events was lower among those who received 0.5 mg of colchicine once daily than among those who did not receive colchicine.12 This was an open-label trial involving only 532 patients, and the results required confirmation.
Accordingly, we conducted an investigator-initiated, randomized, controlled, double-blind, event-driven trial of low-dose colchicine (LoDoCo2) to determine whether 0.5 mg of colchicine once daily, as compared with placebo, prevents cardiovascular events in patients with chronic coronary disease.
Trial Design and Oversight
Patient recruitment in the LoDoCo2 trial commenced on August 4, 2014, at 13 centers affiliated with GenesisCare and the Heart and Vascular Research Institute of Sir Charles Gairdner Hospital in Western Australia. On October 27, 2016, patient recruitment was expanded with the inclusion of 30 centers of the Dutch Network for Cardiovascular Research in the Netherlands. Enrollment was completed by December 3, 2018. The design of the trial has been published previ-
ously.13 The trial protocol, available with the full text of this article at NEJM.org, was approved by a centralized institutional review board in each participating country. An independent data and safety monitoring board reviewed cumulative safe-
ty data to safeguard the well-being of the patients. Full details of the trial organization and a list of the trial sites and investigators are provided in the Supplementary Appendix, also available at NEJM.org.
Trial Population
Patients 35 to 82 years of age were eligible if they had any evidence of coronary disease on invasive coronary angiography or computed tomography angiography or a coronary-artery calcium score of at least 400 Agatston units on a coronarartery
calcium scan. Patients were required to have been The academic and clinical investigators designed the study, collected and managed the data, performed the statistical analyses, and drafted the manuscript. The funders had no role in the
design or writing of the protocol and statistical analysis plan; in the selection or monitoring of the participating sites; in the enrollment or follow-up of patients; in the distribution or administration of the trial drug or placebo; in the collection, storage, analysis, and interpretation of the data; in the drafting of the manuscript; or in the decision to submit the manuscript for publication. The trial drug and matching placebo were donated by Aspen Pharmacare in Australia and by Tiofarma in the Netherlands. The members of the steering committee and the trial statisticians had unrestricted access to the data and vouch for the completeness and accuracy of the data and analyses and for the fidelity of the trial to the protocol in a clinically stable condition for at least 6 months before enrollment. Patients were not eligible if they had moderate-to-severe renal impairment, severe heart failure, severe valvular heart disease, or known side effects from colchicine. Renal function was defined on the basis of the Kidney Disease: Improving Global Outcomes (KDIGO)
Clinical Practice Guideline for Acute Kidney Injury.14 A full list of the inclusion and exclusioncriteria is provided in Table S1 in the Supplementary Appendix. All the patients provided written informed consent to participate.
Run-in, Randomization, and Follow-up
After signing the informed-consent form, eligible patients entered an open-label run-in phase for 1 month, during which time they received 0.5 mg of colchicine once daily. At the end of the open label run-in phase, the patients who were in sta-
ble condition and had no unacceptable side effects, had adhered to the open-label colchicine regimen, and remained willing to continue participation were randomly assigned in a 1:1 ratio to receive 0.5 mg of colchicine once daily or matching pla-
cebo. Randomization was performed in a doubleblind manner with the use of a computerized algorithm, with stratification according to country. Clinical evaluations were scheduled before the run-in phase, at the time of randomization, and at 6-month intervals until the completion of the trial. All follow-up assessments were performed in person, if possible, or by telephone. The trial regimens were continued until the completion of the trial. Moreover, clinical follow-up was con-
tinued until the date of trial completion regardless of premature discontinuation of colchicine or placebo.
End Points
The primary end point was a composite of cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization. Secondary end points, which were tested in hierarchical
fashion, were ranked in the following order: the composite of cardiovascular death, spontaneous myocardial infarction, or ischemic stroke (key secondary end point); the composite of spontaneous myocardial infarction or ischemia-driven
coronary revascularization; the composite of cardiovascular death or spontaneous myocardial in farction; ischemia-driven coronary revascularization; spontaneous myocardial infarction; ischemic stroke; death from any cause; and cardiovascular
death. The list of end points, including the primary end point, was revised several times during the trial; the latest and final revision took place in January 2020 before the data were unblinded. End points were adjudicated by a committee whose
members were unaware of the trial-group assignments. Additional end points and definitions are provided in Table S2.
Statistical Analysis
The trial was designed to accrue a minimum of 331 primary end-point events and to have a minimum follow-up of 1 year. On the basis of a target enrollment of 6053 patients in the open-label run-in phase, with 5447 undergoing randomization after screening, we estimated that the trial would have more than 90% power, at a two-sided alpha level of 0.05, to detect a 30% lower rate (i.e., a hazard ratio of 0.70) of a primary composite end-point event in the colchicine group than in the placebo group, assuming a 10% rate of discontinuation of colchicine or placebo and an annual rate of the primary end point in the control group of 2.6%. Details of the statistical methods are provided in the Supplementary Appendix. The main analysis was based on the time from randomization to the first occurrence of any component of the primary composite end point. If the incidence of the primary end point was significantly lower in the colchicine group than in the placebo group (P<0.05), then the ranked secondary end points were tested in a hierarchical fashion at a significance level of 0.05 in order to preserve the alpha level. The original protocol did not include a plan to adjust for multiple testing; hierarchical testing was included in the protocol in January 2020 before the data were unblinded to be consistent with the new guidelines for statistical reporting in the Journal. 15
The main analysis was performed according to the intention-to-treat principle and included all adjudicated end-point events that occurred between randomization and the end-of-trial date in all patients who had undergone randomization, regardless of whether they adhered to their assigned regimen. Cause-specific hazard ratios in the colchicine group, as compared with the placebo group, and 95% confidence intervals were determined with the use of Cox proportional-hazards models, stratified according to country. If an end-point event had not occurred, follow-up data were censored at the time of the competing risk event (death from noncardiovascular causes or death from any cause, as appropriate) or at the end of the trial. Two-sided P values for superiority
were calculated with the use of log-rank tests, as governed by the rules of hierarchical testing. The prespecified subgroup analyses were performed
with the use of the Cox proportional-hazards method.An exploratory sensitivity analysis of the primary end point in the on-treatment data set was also performed. The on-treatment analysis was performed in patients who had received at least one dose of colchicine or placebo, with additional censoring of data 30 days after the last dose was received (in addition to the data that were censored according to the rules for the main intention-to-treat analysis). Analyses of the primary and secondary end points were also performed with the use of Fine and Gray subdistribution hazard models to account for competing risks.
RESULTS
Patients
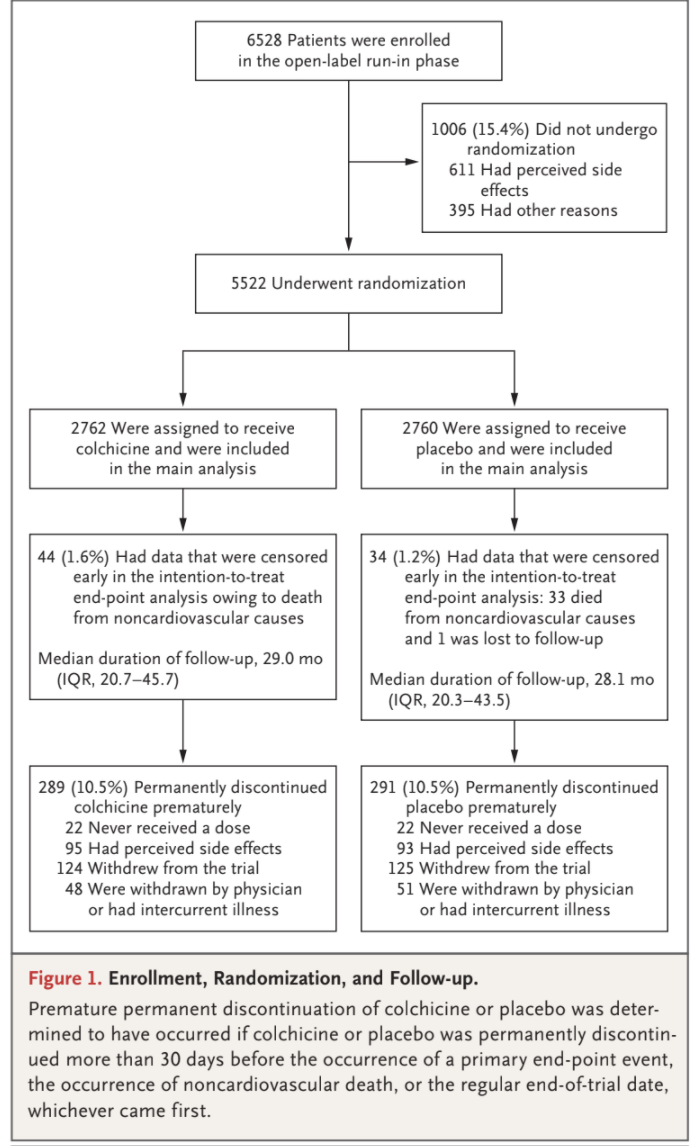
Among the 6528 patients who provided written informed consent and started the open-label run-in period, 5522 underwent randomization and
5478 received at least one dose of colchicine or placebo (Fig. 1 and Table S3). Among the 1006 patients (15.4%) who had started the run-in period
but did not undergo randomization, the most common reason was gastrointestinal upset (in 437 patients). The baseline characteristics of the patients were well balanced between the trial groups (Table 1). The mean (±SD) age of the patients was 66±8.6 years, and 846 (15.3%) were female; 11.7% of the patients were current smokers, and 18.2% had diabetes. Most patients (4658 [84.4%]) had a history of acute coronary syndrome; in 68.2% of the patients, the acute event had occurred more
than 24 months before randomization. At baseline, the patients were well treated with respect to chronic coronary disease, with 99.7% taking an
antiplatelet agent or an anticoagulant, 96.6% a lipid-lowering agent, 62.1% a beta-blocker, and 71.7% an inhibitor of the renin–angiotensin system. Distribution of baseline characteristics according to country is provided in Table S4.
Adherence and Follow-up
The date of the last follow-up contact with a patient was February 17, 2020. The database was locked on May 22, 2020. The primary end-point
status was available for all but one patient. The median duration of follow-up was 28.6 months (interquartile range, 20.5 to 44.4). In each trial
group, 10.5% of the patients permanently discontinued colchicine or placebo prematurely (Fig. 1).
Primary and Secondary End Points
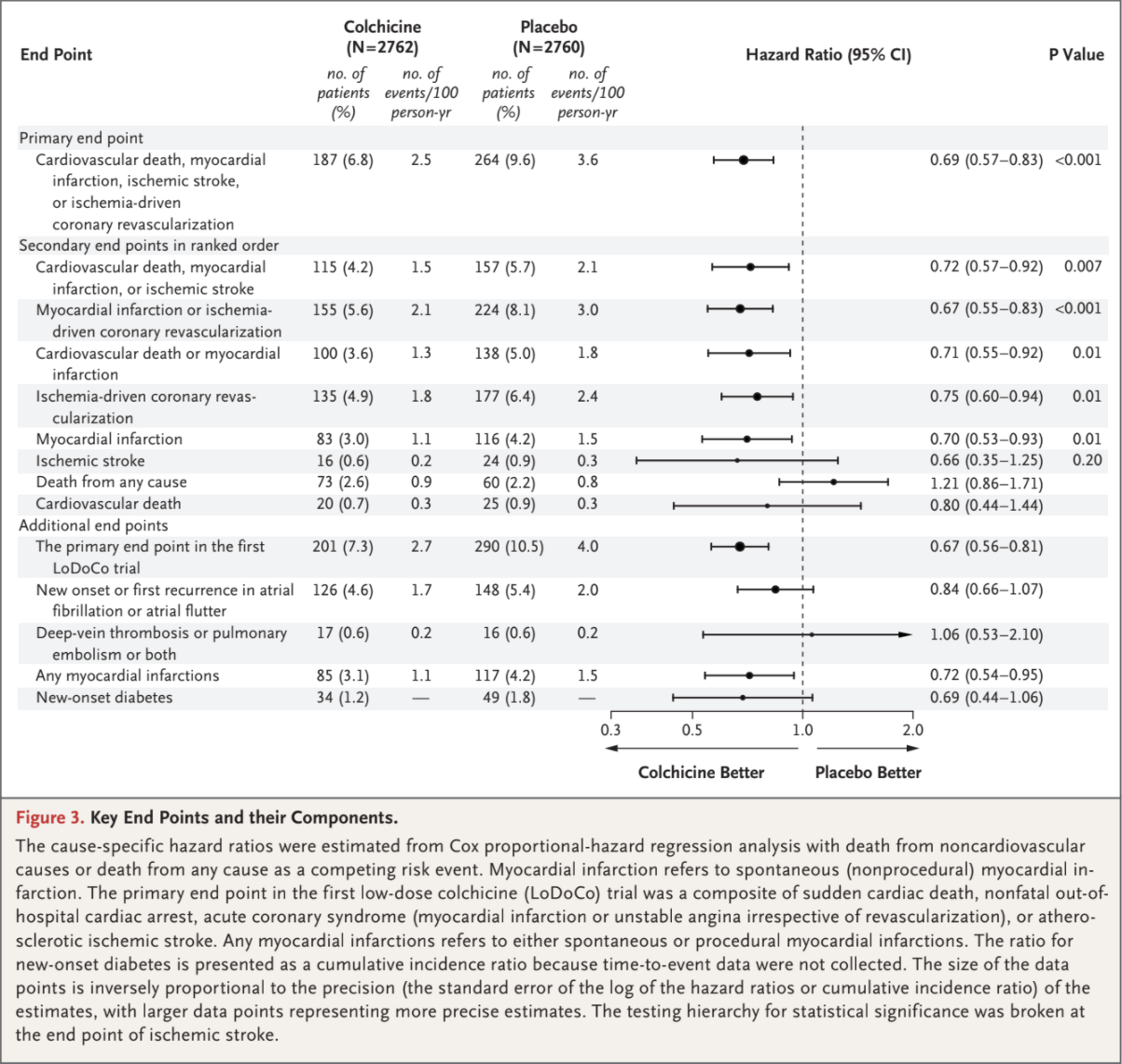
The primary composite end-point event of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization occurred in 187 patients (6.8%) in the colchicine group and in 264 patients (9.6%)
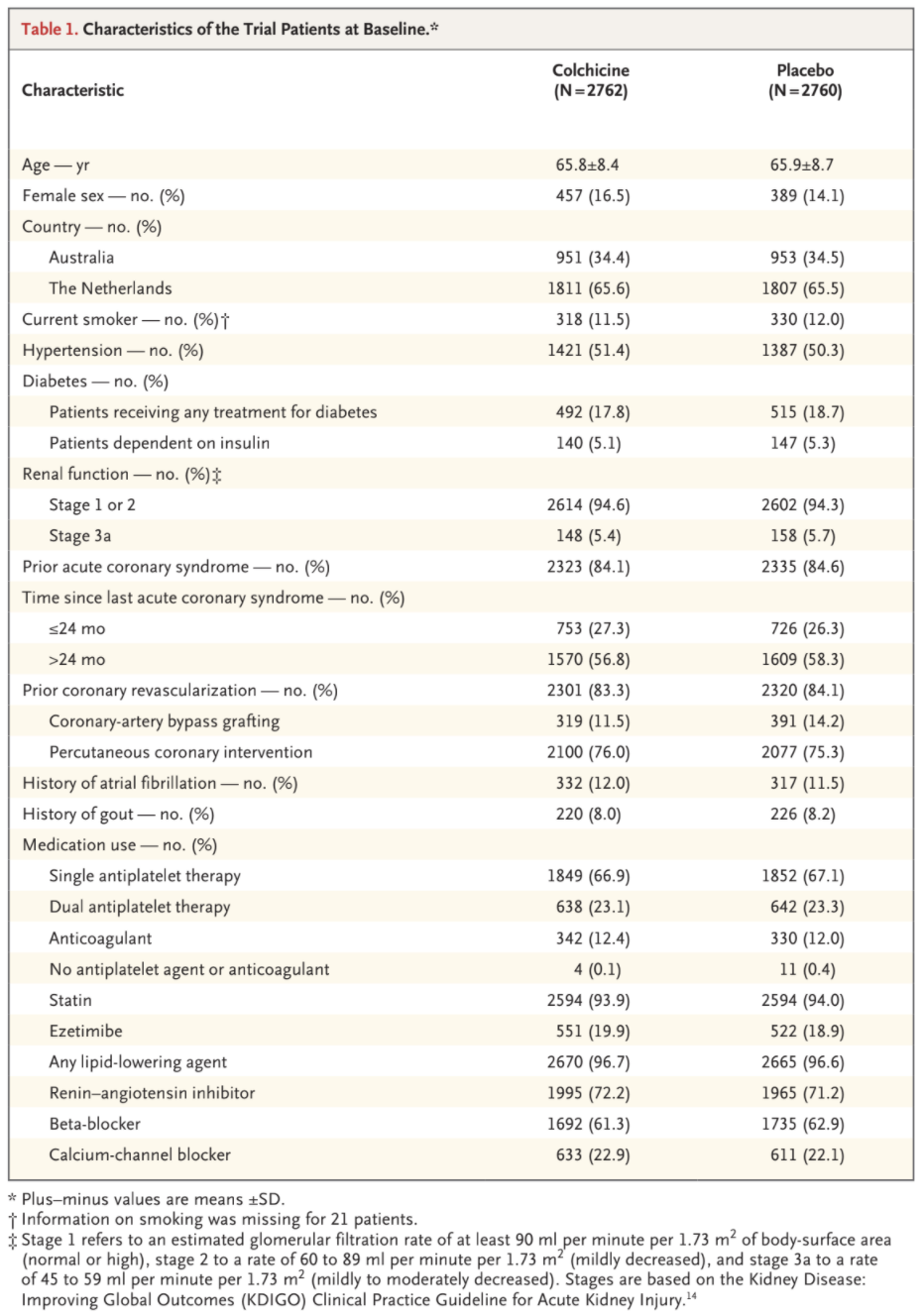
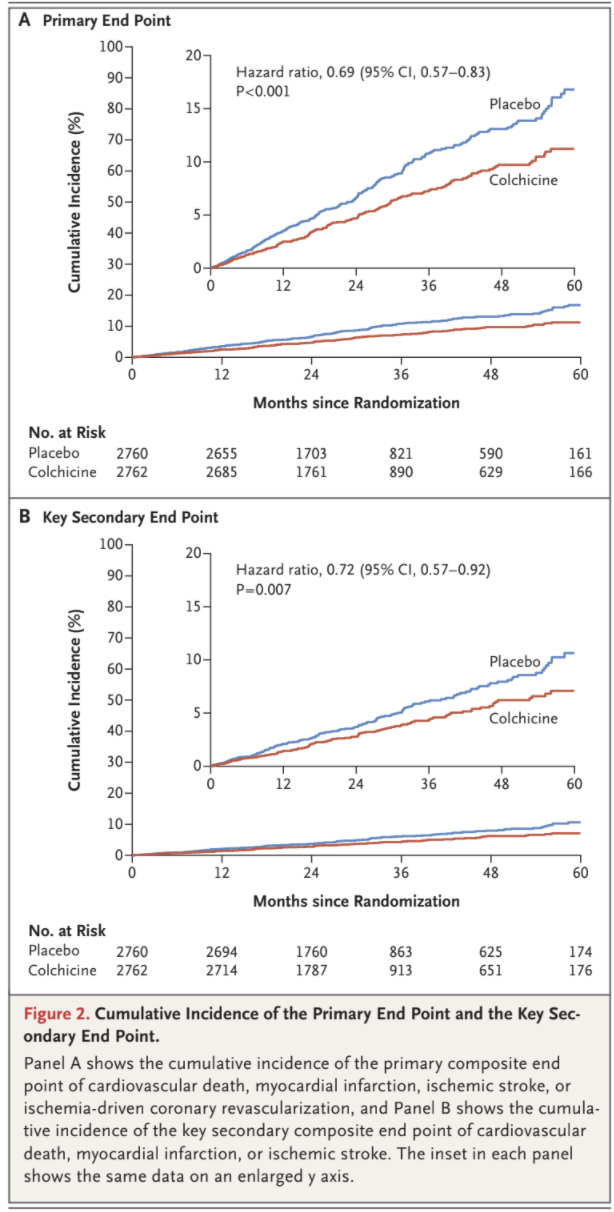
in the placebo group, with incidence rates of 2.5 and 3.6 events, respectively, per 100 person-years (hazard ratio, 0.69; 95% confidence interval [CI], 0.57 to 0.83; P<0.001) (Figs. 2 and 3). This treatment effect was consistent in the on-treatment analysis (Fig. S1 and Table S5).
A key secondary composite end-point event of cardiovascular death, spontaneous myocardial infarction, or ischemic stroke occurred in 115 pa-
tients (4.2%) in the colchicine group and in 157 patients (5.7%) in the placebo group, with incidence rates of 1.5 and 2.1 events, respectively, per 100 person-years (hazard ratio, 0.72; 95% CI, 0.57 to 0.92; P=0.007) (Figs. 2 and 3). In the prespecified hierarchical testing of the ranked sec-
ondary end points, the rates of the first five secondary end points, including spontaneous myocardial infarction, were significantly lower in the
colchicine group than in the placebo group (Fig. 3). Colchicine did not result in a lower incidence of death from any cause than placebo
(73 vs. 60 fatalities; incidence, 0.9 vs. 0.8 events, respectively, per 100 person-years; hazard ratio, 1.21; 95% CI, 0.86 to 1.71). Fine and Gray subdistribution hazard ratios were virtually identical to the cause-specific hazard ratios (Table S6.)
Additional End Points
A primary composite end-point event as defined in the first LoDoCo trial (sudden cardiac death, nonfatal out-of-hospital cardiac arrest, acute coro-
nary syndrome [myocardial infarction or unstable angina irrespective of revascularization], or atherosclerotic ischemic stroke) occurred in 201 pa-
tients in the colchicine group and in 290 patients in the placebo group, with incidence rates of 2.7 and 4.0 events, respectively, per 100 person-years (hazard ratio, 0.67; 95% CI, 0.56 to 0.81) (Fig. 3). The results with respect to the occurrence of new onset or first recurrence of atrial fibrillation, deep-venous thrombosis or pulmonary embolism or both, and new-onset diabetes did not differ significantly between the trial groups.
Adverse Events
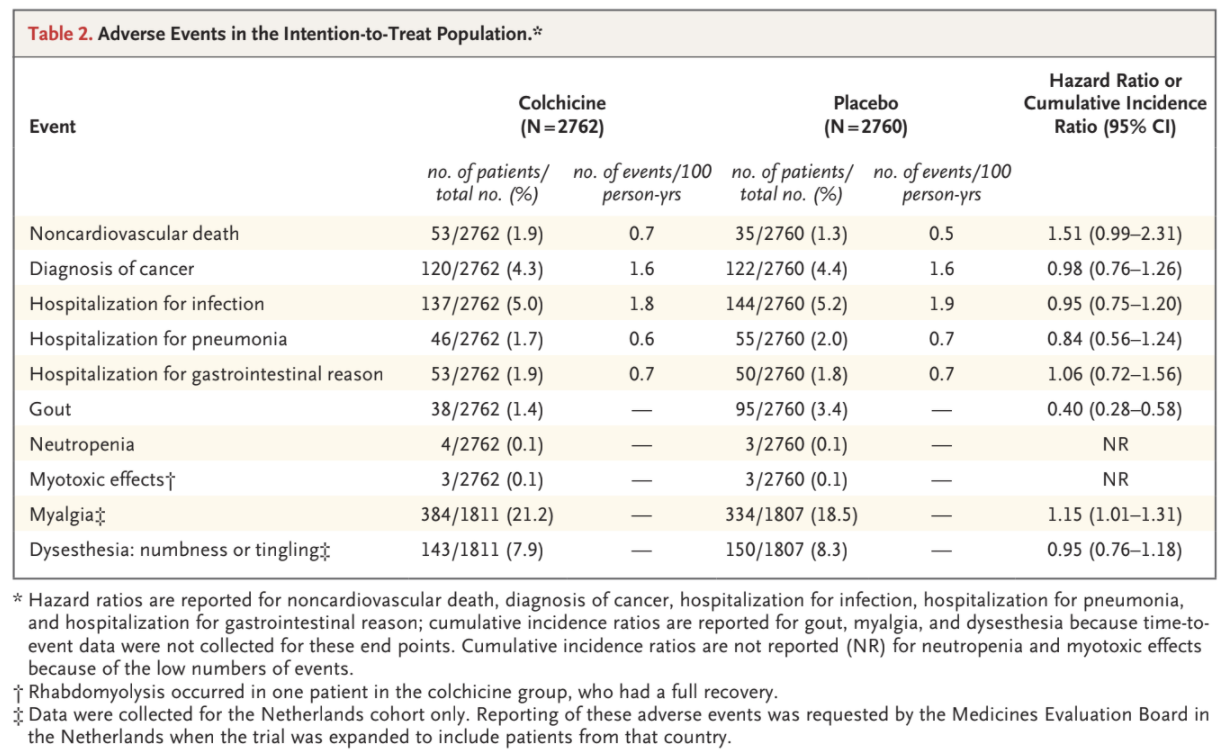
Noncardiovascular deaths occurred more frequently among the patients who received colchicine than among those who received placebo, with incidence rates of 0.7 and 0.5 events, respectively, per 100 person-years (hazard ratio, 1.51; 95% CI, 0.99 to 2.31) (Table 2 and Table S7). We
observed similar rates of cancer diagnosis, hospitalization for infection, hospitalization for pneumonia, and hospitalization for a gastrointestinal
reason in the two trial groups, in both the intention-to-treat analysis and the on-treatment analysis (Table 2 and Table S8). Gout occurred in 38
patients (1.4%) in the colchicine group and in 95 patients (3.4%) in the placebo group (cumulative incidence ratio, 0.40; 95% CI, 0.28 to 0.58). Neutropenia and myotoxic effects were uncommon in both trial groups. Among the patients from the Netherlands, myalgia was reported in 384 (21.2%) in the colchicine group and 334 (18.5%) in the placebo group (cumulative incidence ratio, 1.15; 95% CI, 1.01 to 1.31). Dysesthesia was reported in 143 patients (7.9%) in the colchicine group and in 150 patients (8.3%) in the placebo group (cumulative incidence ratio, 0.95; 95% CI, 0.76 to 1.18).
Subgroup Analyses
The effects of colchicine, as compared with placebo, on the primary end point were consistent in the prespecified subgroups defined according to
sex, age (>65 years vs. ≤65 years), smoking status (current vs. former or never), hypertension (yes vs. no), diabetes (yes vs. no), renal function (stage 1 or 2 vs. stage 3a [stages are based on the KDIGO Clinical Practice Guideline for Acute Kidney Injury14]), prior acute coronary syndrome (yes vs. no), prior coronary revascularization (yes vs. no), atrial fibrillation (yes vs. no), statin dose (high dose vs. low or moderate dose), and ezetimibe use (yes vs. no). When examined according to country, the effect of colchicine, as compared with placebo, on the primary end point was directionally consistent but appeared to be quantitatively larger in Australia than in the Netherlands (Fig. S2).
DISCUSSION
Among patients with chronic coronary disease, most of whom were already receiving proven secondary prevention therapies, 0.5 mg of colchicine once daily resulted in a 31% lower relative risk of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven
coronary revascularization (the primary end point) than placebo, with a hazard ratio of 0.69. The effects of colchicine appeared to be consistent
across each component of the primary end point and all secondary composite end points. The incidence rates of death from any cause and
noncardiovascular death were higher with colchicine than with placebo. The observed between group difference in the incidence of noncardio-
vascular death was not significant, as shown by the 95% confidence interval, and could have been due to chance, although the hazard ratio of 1.51 is of potential concern. The individual causes of death (Table S7) do not permit a clear interpretation of this finding. In the COLCOT trial, non-
cardiovascular death occurred in 23 patients who received colchicine and in 20 patients who received placebo.11
Among the patients who were enrolled in the run-in phase, 15.4% did not undergo randomization; the most common reason was gastrointestinal upset. Among the patients who had successfully completed the run-in phase and had undergone randomization, 10.5% in each trial group permanently discontinued colchicine or placebo prematurely. Our results provide no evidence for a clinically important interaction between low-dose colchicine and high-dose statins, which were used by 3413 patients (61.8%) in the trial. Myalgia, which was assessed only in the Netherlands cohort, was common in both trial groups, although it was reported more frequently in the colchicine group.
The CANTOS trial provided evidence suggesting that inflammation plays a causal role in the pathogenesis of cardiovascular disease and related complications and that interventions to mitigate inflammation may reduce the risk of cardiovascular events.6
Our results with colchicine are consistent with those obtained in the first
LoDoCo trial and the COLCOT trial and provide further support for the potential benefits of antiinflammatory therapy in patients with coronary
disease.11,12 The magnitude of benefit of low-dose colchicine in the LoDoCo2 trial is consistent with that shown in previous trials of antiinflammatory therapy and in previous trials of other secondary prevention therapies, including lipid-lowering, blood pressure–lowering, and antithrombotic therapies, and a benefit was observed in the
patients who were already receiving therapy with these agents.1,3,16-18 Furthermore, the benefits emerged early and continued to accrue through-
out the trial, with no suggestion of attenuation of benefit during up to 5 years of treatment. The LoDoCo2 trial has several limitations.
The percentage of women in the trial was lower than would be expected given the percentage of women with chronic coronary disease in the gen-
eral population. We did not collect blood-pressure or lipid levels at baseline or during the trial, and we cannot report outcomes according to risk-
factor control. We did not routinely measure C-reactive protein levels or other laboratory indicators of inflammation at baseline, and we can-
not explore the effects of treatment according to inflammatory state at baseline. However, the effects of treatments were consistent across the
majority of clinical subgroups examined.
The results of our trial show that among patients with chronic coronary disease, most of whom were already receiving proven secondary prevention therapies, the occurrence of cardiovascular events was significantly lower with low-dose colchicine than with placebo.
Supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. A data sharing statement provided by the authors is available
with the full text of this article at NEJM.org. We thank all the patients for their participation in the trial; the trial investigators and coordinators at all the centers; and the trial monitors and staff from GenesisCare, including Penny Buczec, Denny Craig, Karen Doherty, Louise Ferguson, Louise Nidorf, and Karen Youl, from the Heart and Vascular Research Institute of
Sir Charles Gairdner Hospital, including Louise Ferguson, and from the Dutch Network for Cardiovascular Research, including Marjelle van Leeuwen (project manager), Ingrid Groenenberg and Glentino Rodriguez for data management, Erik Stroes, Max Silvis, and Tim de Vries for medical review, and Petra Bunschoten and Wendy Tousain for site monitoring.
Appendix
The authors’ full names and academic degrees are as follows: Stefan M. Nidorf, M.D., Aernoud T.L. Fiolet, M.D., Arend Mosterd, M.D., John W. Eikelboom, M.D., Astrid Schut, M.Sc., Tjerk S.J. Opstal, M.D., Salem H.K. The, M.D., Xiao-Fang Xu, M.D., Mark A. Ireland, M.D., Timo Lenderink, M.D., Donald Latchem, M.D., Pieter Hoogslag, M.D., Anastazia Jerzewski, M.D., Peter Nierop, M.D., Alan Whelan, M.D., Randall Hendriks, M.D., Henk Swart, M.D., Jeroen Schaap, M.D., Aaf F.M. Kuijper, M.D., Maarten W.J. van Hessen, M.D., Pradyot Saklani, M.D., Isabel Tan, M.D., Angus G. Thompson, M.D., Allison Morton, M.D., Chris Judkins, M.D., Willem A. Bax,
M.D., Maurits Dirksen, M.D., Marco M.W. Alings, M.D., Graeme J. Hankey, M.D., Charley A. Budgeon, Ph.D., Jan G.P. Tijssen, Ph.D., Jan H. Cornel, M.D., and Peter L. Thompson, M.D. The authors’ affiliations are as follows: GenesisCare Western Australia (S.M.N., X.-F.X., M.A.I., D.L., A.W., R.H., P.S., I.T., A.G.T., A. Morton, P.L.T.), the Heart and Vascular Research Institute (S.M.N., P.L.T.) and the Department of Neurology (G.J.H.), Sir Charles Gairdner Hospital, and the Faculty of Health and Medical Sciences (G.J.H., P.L.T.) and the School of Population and Global Health
(C.A.B.), University of Western Australia, Perth, the Department of Cardiology, Fiona Stanley Hospital, Murdoch, WA (C.J.), and the
Harry Perkins Institute of Medical Research, Nedlands, WA (P.L.T.) — all in Australia; the Dutch Network for Cardiovascular Research
(A.T.L.F., A. Mosterd, A.S., S.H.K.T., T.L., P.H., A.J., P.N., H.S., J.S., A.F.M.K., M.W.J.H., M.D., M.M.W.A., J.H.C.), the Netherlands
Heart Institute (A.T.L.F.), and the Department of Cardiology (A.T.L.F.) and the Julius Center for Health Sciences and Primary Care (A.
Mosterd, M.M.W.A.), University Medical Center Utrecht, Utrecht, the Department of Cardiology, Meander Medical Center, Amersfoort
(A. Mosterd), the Departments of Cardiology (T.S.J.O., M.D., J.H.C.) and Internal Medicine (W.A.B.), Northwest Clinics, Alkmaar, the
Department of Cardiology, Radboud University Medical Center, Nijmegen (T.S.J.O., J.H.C.), the Department of Cardiology, Treant Zorggroep, Hoogeveen, Emmen, and Stadskanaal (S.H.K.T.), the Department of Cardiology, Zuyderland Medical Center, Heerlen and Sittard
(T.L.), the Department of Cardiology, Isala Diaconessenhuis, Meppel (P.H.), the Department of Cardiology, Gelre Hospitals, Apeldoorn
(A.J.), the Department of Cardiology, Franciscus Hospital (P.N.), and Cardialysis (J.G.P.T.), Rotterdam, the Department of Cardiology,
D&A Research and Genetics, Sneek (H.S.), the Department of Cardiology, Amphia and Breda (J.S., M.M.W.A.), the Department of Cardiology, Spaarne Hospital, Haarlem and Hoofddorp (A.F.M.K.), the Department of Cardiology, Green Heart Hospital, Gouda (M.W.J.H.), and the Department of Cardiology, Amsterdam UMC, Amsterdam (J.G.P.T.) — all in the Netherlands; and the Department of Medicine, McMaster University, Hamilton, ON, Canada (J.W.E.).
References
1. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713-22.
2. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N EnglJ Med 2019;380:11-22.
3. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319-30.
4. Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med 1999;340:115-26.
5. Libby P, Loscalzo J, Ridker PM, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol 2018;72:2071- 81.
6. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with
canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-31.
7. Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752-62.
8. Leung YY, Hui LLY, Kraus VB. Colchicine — update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015;45:341-50.
9. Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther 2014;36: 1465-79.
10. Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest 1995;96:994-1002.
11. Tardif J-C, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497-505.
12. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404-10.
13. Nidorf SM, Fiolet ATL, Eikelboom JW, et al. The effect of low-dose colchicine in patients with stable coronary artery disease: the LoDoCo2 trial rationale, design, and baseline characteristics. Am Heart J 2019;218:46-56.
14. Kellum JA, Lameire N, Aspelin P, et al. Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1-138.
15. Harrington D, D’Agostino RB Sr, Gatsonis C, et al. New guidelines for statistical reporting in the Journal. N Engl J Med 2019;381:285-6.
16. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomisedtrials. Lancet 2010;376:1670-81.
17. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957-67.
18. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and
secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
Copyright © 2020 Massachusetts Medical Society.
Evidence from a recent trial has shown that the antiinflammatory effects of colchicine reduce the risk of cardiovascular events in patients with recent myocardial infarction, but evidence of such a risk reduction in patients with chronic coronary
disease is limited.
METHODS
In a randomized, controlled, double-blind trial, we assigned patients with chronic coronary disease to receive 0.5 mg of colchicine once daily or matching placebo.
The primary end point was a composite of cardiovascular death, spontaneous (non-procedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization. The key secondary end point was a composite of cardiovascular death, spontaneous myocardial infarction, or ischemic stroke.
RESULTS
A total of 5522 patients underwent randomization; 2762 were assigned to the colchicine group and 2760 to the placebo group. The median duration of follow-up was 28.6 months. A primary end-point event occurred in 187 patients (6.8%) in the colchicine group and in 264 patients (9.6%) in the placebo group (incidence, 2.5 vs. 3.6 events per 100 person-years; hazard ratio, 0.69; 95% confidence interval [CI], 0.57 to 0.83; P<0.001). A key secondary end-point event occurred in 115 patients (4.2%) in the colchicine group and in 157 patients (5.7%) in the placebo group (incidence, 1.5 vs. 2.1 events per 100 person-years; hazard ratio, 0.72; 95% CI, 0.57 to 0.92; P=0.007). The incidence rates of spontaneous myocardial infarction or ischemia-driven coronary revascularization (composite end point), cardiovascular death or spontaneous myocardial infarction (composite end point), ischemia-driven coronary revascularization, and spontaneous myocardial infarction were also significantly lower with colchicine than with placebo. The incidence of death from noncardiovascular causes was higher in the colchicine group than in the placebo group (incidence, 0.7 vs. 0.5 events per 100 person-years; hazard ratio, 1.51; 95% CI, 0.99 to 2.31).
CONCLUSIONS
In a randomized trial involving patients with chronic coronary disease, the risk of cardiovascular events was significantly lower among those who received 0.5 mg of colchicine once daily than among those who received placebo. (Funded by the National Health Medical Research Council of Australia and others; LoDoCo2 Australian New Zealand Clinical Trials Registry number, ACTRN12614000093684.)
Despite lifestyle changes and risk factor reduction, patients with chronic coronary disease remain at high risk for
acute cardiovascular events.1-3 The central role of inflammation in the progression of coronary disease is well recognized.4,5 The possibility that antiinflammatory therapy may improve cardiovascular outcomes was first highlighted in the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) involving patients with a history of myocardial infarction and an elevated baseline level of C-reactive protein; the results showed that the risk of recurrent cardiovascular
events was lower among those who received canakinumab than among those who received placebo.6
However, in another trial, a clinical benefit with methotrexate was not observed in patients with chronic coronary disease.7
Colchicine is an antiinflammatory drug originally extracted from the autumn crocus (Colchicum autumnale) and was used by the ancient Greeks and Egyptians. In contrast to selective inhibition of interleukin-1β by canakinumab, colchicine
has broad cellular effects that include inhibition of tubulin polymerization and alteration of leukocyte responsiveness.8-10 In the Colchicine Cardiovascular Outcomes Trial (COLCOT) involving patients who had a myocardial infarction within
30 days before enrollment, the percentage of those who had the composite end point of cardiovascular death, resuscitated cardiac arrest, myocardial infarction, stroke, or urgent hospitalization for angina leading to coronary revascularization was
lower among those who received 0.5 mg of colchicine once daily than among those who received placebo.11
In an earlier trial of low-dose colchicine (LoDoCo) involving patients with chronic coronary disease, we found that the risk of acute cardiovascular events was lower among those who received 0.5 mg of colchicine once daily than among those who did not receive colchicine.12 This was an open-label trial involving only 532 patients, and the results required confirmation.
Accordingly, we conducted an investigator-initiated, randomized, controlled, double-blind, event-driven trial of low-dose colchicine (LoDoCo2) to determine whether 0.5 mg of colchicine once daily, as compared with placebo, prevents cardiovascular events in patients with chronic coronary disease.
Trial Design and Oversight
Patient recruitment in the LoDoCo2 trial commenced on August 4, 2014, at 13 centers affiliated with GenesisCare and the Heart and Vascular Research Institute of Sir Charles Gairdner Hospital in Western Australia. On October 27, 2016, patient recruitment was expanded with the inclusion of 30 centers of the Dutch Network for Cardiovascular Research in the Netherlands. Enrollment was completed by December 3, 2018. The design of the trial has been published previ-
ously.13 The trial protocol, available with the full text of this article at NEJM.org, was approved by a centralized institutional review board in each participating country. An independent data and safety monitoring board reviewed cumulative safe-
ty data to safeguard the well-being of the patients. Full details of the trial organization and a list of the trial sites and investigators are provided in the Supplementary Appendix, also available at NEJM.org.
Trial Population
Patients 35 to 82 years of age were eligible if they had any evidence of coronary disease on invasive coronary angiography or computed tomography angiography or a coronary-artery calcium score of at least 400 Agatston units on a coronarartery
calcium scan. Patients were required to have been The academic and clinical investigators designed the study, collected and managed the data, performed the statistical analyses, and drafted the manuscript. The funders had no role in the
design or writing of the protocol and statistical analysis plan; in the selection or monitoring of the participating sites; in the enrollment or follow-up of patients; in the distribution or administration of the trial drug or placebo; in the collection, storage, analysis, and interpretation of the data; in the drafting of the manuscript; or in the decision to submit the manuscript for publication. The trial drug and matching placebo were donated by Aspen Pharmacare in Australia and by Tiofarma in the Netherlands. The members of the steering committee and the trial statisticians had unrestricted access to the data and vouch for the completeness and accuracy of the data and analyses and for the fidelity of the trial to the protocol in a clinically stable condition for at least 6 months before enrollment. Patients were not eligible if they had moderate-to-severe renal impairment, severe heart failure, severe valvular heart disease, or known side effects from colchicine. Renal function was defined on the basis of the Kidney Disease: Improving Global Outcomes (KDIGO)
Clinical Practice Guideline for Acute Kidney Injury.14 A full list of the inclusion and exclusioncriteria is provided in Table S1 in the Supplementary Appendix. All the patients provided written informed consent to participate.
Run-in, Randomization, and Follow-up
After signing the informed-consent form, eligible patients entered an open-label run-in phase for 1 month, during which time they received 0.5 mg of colchicine once daily. At the end of the open label run-in phase, the patients who were in sta-
ble condition and had no unacceptable side effects, had adhered to the open-label colchicine regimen, and remained willing to continue participation were randomly assigned in a 1:1 ratio to receive 0.5 mg of colchicine once daily or matching pla-
cebo. Randomization was performed in a doubleblind manner with the use of a computerized algorithm, with stratification according to country. Clinical evaluations were scheduled before the run-in phase, at the time of randomization, and at 6-month intervals until the completion of the trial. All follow-up assessments were performed in person, if possible, or by telephone. The trial regimens were continued until the completion of the trial. Moreover, clinical follow-up was con-
tinued until the date of trial completion regardless of premature discontinuation of colchicine or placebo.
End Points
The primary end point was a composite of cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization. Secondary end points, which were tested in hierarchical
fashion, were ranked in the following order: the composite of cardiovascular death, spontaneous myocardial infarction, or ischemic stroke (key secondary end point); the composite of spontaneous myocardial infarction or ischemia-driven
coronary revascularization; the composite of cardiovascular death or spontaneous myocardial in farction; ischemia-driven coronary revascularization; spontaneous myocardial infarction; ischemic stroke; death from any cause; and cardiovascular
death. The list of end points, including the primary end point, was revised several times during the trial; the latest and final revision took place in January 2020 before the data were unblinded. End points were adjudicated by a committee whose
members were unaware of the trial-group assignments. Additional end points and definitions are provided in Table S2.
Statistical Analysis
The trial was designed to accrue a minimum of 331 primary end-point events and to have a minimum follow-up of 1 year. On the basis of a target enrollment of 6053 patients in the open-label run-in phase, with 5447 undergoing randomization after screening, we estimated that the trial would have more than 90% power, at a two-sided alpha level of 0.05, to detect a 30% lower rate (i.e., a hazard ratio of 0.70) of a primary composite end-point event in the colchicine group than in the placebo group, assuming a 10% rate of discontinuation of colchicine or placebo and an annual rate of the primary end point in the control group of 2.6%. Details of the statistical methods are provided in the Supplementary Appendix. The main analysis was based on the time from randomization to the first occurrence of any component of the primary composite end point. If the incidence of the primary end point was significantly lower in the colchicine group than in the placebo group (P<0.05), then the ranked secondary end points were tested in a hierarchical fashion at a significance level of 0.05 in order to preserve the alpha level. The original protocol did not include a plan to adjust for multiple testing; hierarchical testing was included in the protocol in January 2020 before the data were unblinded to be consistent with the new guidelines for statistical reporting in the Journal. 15
The main analysis was performed according to the intention-to-treat principle and included all adjudicated end-point events that occurred between randomization and the end-of-trial date in all patients who had undergone randomization, regardless of whether they adhered to their assigned regimen. Cause-specific hazard ratios in the colchicine group, as compared with the placebo group, and 95% confidence intervals were determined with the use of Cox proportional-hazards models, stratified according to country. If an end-point event had not occurred, follow-up data were censored at the time of the competing risk event (death from noncardiovascular causes or death from any cause, as appropriate) or at the end of the trial. Two-sided P values for superiority
were calculated with the use of log-rank tests, as governed by the rules of hierarchical testing. The prespecified subgroup analyses were performed
with the use of the Cox proportional-hazards method.An exploratory sensitivity analysis of the primary end point in the on-treatment data set was also performed. The on-treatment analysis was performed in patients who had received at least one dose of colchicine or placebo, with additional censoring of data 30 days after the last dose was received (in addition to the data that were censored according to the rules for the main intention-to-treat analysis). Analyses of the primary and secondary end points were also performed with the use of Fine and Gray subdistribution hazard models to account for competing risks.

RESULTS
Patients
Among the 6528 patients who provided written informed consent and started the open-label run-in period, 5522 underwent randomization and
5478 received at least one dose of colchicine or placebo (Fig. 1 and Table S3). Among the 1006 patients (15.4%) who had started the run-in period
but did not undergo randomization, the most common reason was gastrointestinal upset (in 437 patients). The baseline characteristics of the patients were well balanced between the trial groups (Table 1). The mean (±SD) age of the patients was 66±8.6 years, and 846 (15.3%) were female; 11.7% of the patients were current smokers, and 18.2% had diabetes. Most patients (4658 [84.4%]) had a history of acute coronary syndrome; in 68.2% of the patients, the acute event had occurred more
than 24 months before randomization. At baseline, the patients were well treated with respect to chronic coronary disease, with 99.7% taking an
antiplatelet agent or an anticoagulant, 96.6% a lipid-lowering agent, 62.1% a beta-blocker, and 71.7% an inhibitor of the renin–angiotensin system. Distribution of baseline characteristics according to country is provided in Table S4.
Adherence and Follow-up
The date of the last follow-up contact with a patient was February 17, 2020. The database was locked on May 22, 2020. The primary end-point
status was available for all but one patient. The median duration of follow-up was 28.6 months (interquartile range, 20.5 to 44.4). In each trial
group, 10.5% of the patients permanently discontinued colchicine or placebo prematurely (Fig. 1).
Primary and Secondary End Points
The primary composite end-point event of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization occurred in 187 patients (6.8%) in the colchicine group and in 264 patients (9.6%)


in the placebo group, with incidence rates of 2.5 and 3.6 events, respectively, per 100 person-years (hazard ratio, 0.69; 95% confidence interval [CI], 0.57 to 0.83; P<0.001) (Figs. 2 and 3). This treatment effect was consistent in the on-treatment analysis (Fig. S1 and Table S5).
A key secondary composite end-point event of cardiovascular death, spontaneous myocardial infarction, or ischemic stroke occurred in 115 pa-
tients (4.2%) in the colchicine group and in 157 patients (5.7%) in the placebo group, with incidence rates of 1.5 and 2.1 events, respectively, per 100 person-years (hazard ratio, 0.72; 95% CI, 0.57 to 0.92; P=0.007) (Figs. 2 and 3). In the prespecified hierarchical testing of the ranked sec-
ondary end points, the rates of the first five secondary end points, including spontaneous myocardial infarction, were significantly lower in the
colchicine group than in the placebo group (Fig. 3). Colchicine did not result in a lower incidence of death from any cause than placebo
(73 vs. 60 fatalities; incidence, 0.9 vs. 0.8 events, respectively, per 100 person-years; hazard ratio, 1.21; 95% CI, 0.86 to 1.71). Fine and Gray subdistribution hazard ratios were virtually identical to the cause-specific hazard ratios (Table S6.)
Additional End Points
A primary composite end-point event as defined in the first LoDoCo trial (sudden cardiac death, nonfatal out-of-hospital cardiac arrest, acute coro-
nary syndrome [myocardial infarction or unstable angina irrespective of revascularization], or atherosclerotic ischemic stroke) occurred in 201 pa-
tients in the colchicine group and in 290 patients in the placebo group, with incidence rates of 2.7 and 4.0 events, respectively, per 100 person-years (hazard ratio, 0.67; 95% CI, 0.56 to 0.81) (Fig. 3). The results with respect to the occurrence of new onset or first recurrence of atrial fibrillation, deep-venous thrombosis or pulmonary embolism or both, and new-onset diabetes did not differ significantly between the trial groups.
Adverse Events
Noncardiovascular deaths occurred more frequently among the patients who received colchicine than among those who received placebo, with incidence rates of 0.7 and 0.5 events, respectively, per 100 person-years (hazard ratio, 1.51; 95% CI, 0.99 to 2.31) (Table 2 and Table S7). We
observed similar rates of cancer diagnosis, hospitalization for infection, hospitalization for pneumonia, and hospitalization for a gastrointestinal
reason in the two trial groups, in both the intention-to-treat analysis and the on-treatment analysis (Table 2 and Table S8). Gout occurred in 38
patients (1.4%) in the colchicine group and in 95 patients (3.4%) in the placebo group (cumulative incidence ratio, 0.40; 95% CI, 0.28 to 0.58). Neutropenia and myotoxic effects were uncommon in both trial groups. Among the patients from the Netherlands, myalgia was reported in 384 (21.2%) in the colchicine group and 334 (18.5%) in the placebo group (cumulative incidence ratio, 1.15; 95% CI, 1.01 to 1.31). Dysesthesia was reported in 143 patients (7.9%) in the colchicine group and in 150 patients (8.3%) in the placebo group (cumulative incidence ratio, 0.95; 95% CI, 0.76 to 1.18).
Subgroup Analyses
The effects of colchicine, as compared with placebo, on the primary end point were consistent in the prespecified subgroups defined according to
sex, age (>65 years vs. ≤65 years), smoking status (current vs. former or never), hypertension (yes vs. no), diabetes (yes vs. no), renal function (stage 1 or 2 vs. stage 3a [stages are based on the KDIGO Clinical Practice Guideline for Acute Kidney Injury14]), prior acute coronary syndrome (yes vs. no), prior coronary revascularization (yes vs. no), atrial fibrillation (yes vs. no), statin dose (high dose vs. low or moderate dose), and ezetimibe use (yes vs. no). When examined according to country, the effect of colchicine, as compared with placebo, on the primary end point was directionally consistent but appeared to be quantitatively larger in Australia than in the Netherlands (Fig. S2).


DISCUSSION
Among patients with chronic coronary disease, most of whom were already receiving proven secondary prevention therapies, 0.5 mg of colchicine once daily resulted in a 31% lower relative risk of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven
coronary revascularization (the primary end point) than placebo, with a hazard ratio of 0.69. The effects of colchicine appeared to be consistent
across each component of the primary end point and all secondary composite end points. The incidence rates of death from any cause and
noncardiovascular death were higher with colchicine than with placebo. The observed between group difference in the incidence of noncardio-
vascular death was not significant, as shown by the 95% confidence interval, and could have been due to chance, although the hazard ratio of 1.51 is of potential concern. The individual causes of death (Table S7) do not permit a clear interpretation of this finding. In the COLCOT trial, non-
cardiovascular death occurred in 23 patients who received colchicine and in 20 patients who received placebo.11
Among the patients who were enrolled in the run-in phase, 15.4% did not undergo randomization; the most common reason was gastrointestinal upset. Among the patients who had successfully completed the run-in phase and had undergone randomization, 10.5% in each trial group permanently discontinued colchicine or placebo prematurely. Our results provide no evidence for a clinically important interaction between low-dose colchicine and high-dose statins, which were used by 3413 patients (61.8%) in the trial. Myalgia, which was assessed only in the Netherlands cohort, was common in both trial groups, although it was reported more frequently in the colchicine group.
The CANTOS trial provided evidence suggesting that inflammation plays a causal role in the pathogenesis of cardiovascular disease and related complications and that interventions to mitigate inflammation may reduce the risk of cardiovascular events.6
Our results with colchicine are consistent with those obtained in the first
LoDoCo trial and the COLCOT trial and provide further support for the potential benefits of antiinflammatory therapy in patients with coronary
disease.11,12 The magnitude of benefit of low-dose colchicine in the LoDoCo2 trial is consistent with that shown in previous trials of antiinflammatory therapy and in previous trials of other secondary prevention therapies, including lipid-lowering, blood pressure–lowering, and antithrombotic therapies, and a benefit was observed in the
patients who were already receiving therapy with these agents.1,3,16-18 Furthermore, the benefits emerged early and continued to accrue through-
out the trial, with no suggestion of attenuation of benefit during up to 5 years of treatment. The LoDoCo2 trial has several limitations.
The percentage of women in the trial was lower than would be expected given the percentage of women with chronic coronary disease in the gen-
eral population. We did not collect blood-pressure or lipid levels at baseline or during the trial, and we cannot report outcomes according to risk-
factor control. We did not routinely measure C-reactive protein levels or other laboratory indicators of inflammation at baseline, and we can-
not explore the effects of treatment according to inflammatory state at baseline. However, the effects of treatments were consistent across the
majority of clinical subgroups examined.
The results of our trial show that among patients with chronic coronary disease, most of whom were already receiving proven secondary prevention therapies, the occurrence of cardiovascular events was significantly lower with low-dose colchicine than with placebo.
Supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. A data sharing statement provided by the authors is available
with the full text of this article at NEJM.org. We thank all the patients for their participation in the trial; the trial investigators and coordinators at all the centers; and the trial monitors and staff from GenesisCare, including Penny Buczec, Denny Craig, Karen Doherty, Louise Ferguson, Louise Nidorf, and Karen Youl, from the Heart and Vascular Research Institute of
Sir Charles Gairdner Hospital, including Louise Ferguson, and from the Dutch Network for Cardiovascular Research, including Marjelle van Leeuwen (project manager), Ingrid Groenenberg and Glentino Rodriguez for data management, Erik Stroes, Max Silvis, and Tim de Vries for medical review, and Petra Bunschoten and Wendy Tousain for site monitoring.
Appendix
The authors’ full names and academic degrees are as follows: Stefan M. Nidorf, M.D., Aernoud T.L. Fiolet, M.D., Arend Mosterd, M.D., John W. Eikelboom, M.D., Astrid Schut, M.Sc., Tjerk S.J. Opstal, M.D., Salem H.K. The, M.D., Xiao-Fang Xu, M.D., Mark A. Ireland, M.D., Timo Lenderink, M.D., Donald Latchem, M.D., Pieter Hoogslag, M.D., Anastazia Jerzewski, M.D., Peter Nierop, M.D., Alan Whelan, M.D., Randall Hendriks, M.D., Henk Swart, M.D., Jeroen Schaap, M.D., Aaf F.M. Kuijper, M.D., Maarten W.J. van Hessen, M.D., Pradyot Saklani, M.D., Isabel Tan, M.D., Angus G. Thompson, M.D., Allison Morton, M.D., Chris Judkins, M.D., Willem A. Bax,
M.D., Maurits Dirksen, M.D., Marco M.W. Alings, M.D., Graeme J. Hankey, M.D., Charley A. Budgeon, Ph.D., Jan G.P. Tijssen, Ph.D., Jan H. Cornel, M.D., and Peter L. Thompson, M.D. The authors’ affiliations are as follows: GenesisCare Western Australia (S.M.N., X.-F.X., M.A.I., D.L., A.W., R.H., P.S., I.T., A.G.T., A. Morton, P.L.T.), the Heart and Vascular Research Institute (S.M.N., P.L.T.) and the Department of Neurology (G.J.H.), Sir Charles Gairdner Hospital, and the Faculty of Health and Medical Sciences (G.J.H., P.L.T.) and the School of Population and Global Health
(C.A.B.), University of Western Australia, Perth, the Department of Cardiology, Fiona Stanley Hospital, Murdoch, WA (C.J.), and the
Harry Perkins Institute of Medical Research, Nedlands, WA (P.L.T.) — all in Australia; the Dutch Network for Cardiovascular Research
(A.T.L.F., A. Mosterd, A.S., S.H.K.T., T.L., P.H., A.J., P.N., H.S., J.S., A.F.M.K., M.W.J.H., M.D., M.M.W.A., J.H.C.), the Netherlands
Heart Institute (A.T.L.F.), and the Department of Cardiology (A.T.L.F.) and the Julius Center for Health Sciences and Primary Care (A.
Mosterd, M.M.W.A.), University Medical Center Utrecht, Utrecht, the Department of Cardiology, Meander Medical Center, Amersfoort
(A. Mosterd), the Departments of Cardiology (T.S.J.O., M.D., J.H.C.) and Internal Medicine (W.A.B.), Northwest Clinics, Alkmaar, the
Department of Cardiology, Radboud University Medical Center, Nijmegen (T.S.J.O., J.H.C.), the Department of Cardiology, Treant Zorggroep, Hoogeveen, Emmen, and Stadskanaal (S.H.K.T.), the Department of Cardiology, Zuyderland Medical Center, Heerlen and Sittard
(T.L.), the Department of Cardiology, Isala Diaconessenhuis, Meppel (P.H.), the Department of Cardiology, Gelre Hospitals, Apeldoorn
(A.J.), the Department of Cardiology, Franciscus Hospital (P.N.), and Cardialysis (J.G.P.T.), Rotterdam, the Department of Cardiology,
D&A Research and Genetics, Sneek (H.S.), the Department of Cardiology, Amphia and Breda (J.S., M.M.W.A.), the Department of Cardiology, Spaarne Hospital, Haarlem and Hoofddorp (A.F.M.K.), the Department of Cardiology, Green Heart Hospital, Gouda (M.W.J.H.), and the Department of Cardiology, Amsterdam UMC, Amsterdam (J.G.P.T.) — all in the Netherlands; and the Department of Medicine, McMaster University, Hamilton, ON, Canada (J.W.E.).
References
1. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713-22.
2. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N EnglJ Med 2019;380:11-22.
3. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319-30.
4. Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med 1999;340:115-26.
5. Libby P, Loscalzo J, Ridker PM, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol 2018;72:2071- 81.
6. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with
canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-31.
7. Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752-62.
8. Leung YY, Hui LLY, Kraus VB. Colchicine — update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015;45:341-50.
9. Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther 2014;36: 1465-79.
10. Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest 1995;96:994-1002.
11. Tardif J-C, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497-505.
12. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404-10.
13. Nidorf SM, Fiolet ATL, Eikelboom JW, et al. The effect of low-dose colchicine in patients with stable coronary artery disease: the LoDoCo2 trial rationale, design, and baseline characteristics. Am Heart J 2019;218:46-56.
14. Kellum JA, Lameire N, Aspelin P, et al. Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1-138.
15. Harrington D, D’Agostino RB Sr, Gatsonis C, et al. New guidelines for statistical reporting in the Journal. N Engl J Med 2019;381:285-6.
16. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomisedtrials. Lancet 2010;376:1670-81.
17. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957-67.
18. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and
secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
Copyright © 2020 Massachusetts Medical Society.