Λίστα αντικειμένων
Objective:
To analyze and compare the expression profile of TAC3, TACR3, KISS1, and KISS1R in mural granulosa and cumulus cells
from healthy oocyte donors and patients with different infertility etiologies, including advanced maternal age, endometriosis, and low
ovarian response.
Design: Genetic association study.
Setting: Private fertility clinic and public research laboratory.
Patient(s): Healthy oocyte donors and infertile women undergoing in vitro fertilization (IVF) treatment.
Intervention(s): IVF.
Main Outcome Measure(s): Gene expression levels of KISS1, KISS1R, TAC3, and TACR3 in human mural granulosa and cumulus
cells.
Result(s): Infertile women showed statistically significantly altered expression levels of KISS1 (2.57 2.30 vs. 1.37 2.11), TAC3
(1.21 1.40 vs. 1.49 1.98), and TACR3 (0.77 1.36 vs. 0.03 0.56) when compared with healthy oocyte donors. Advanced
maternal age patients, endometriosis patients, and low responders showed specific and altered expression profiles in comparison with
oocyte donors.
Conclusion(s): Abnormal expression levels of KISS1/KISS1R and TAC3/TACR3 systems in granulosa cells might be involved in the
decreased fertility associated to advanced maternal age, endometriosis, and low ovarian response. (Fertil Steril 2020;114:869-78.
2020 by American Society for Reproductive Medicine.)
El resumen está disponible en Español al final del artículo.
Key Words: #GranulosaCells, #humanInfertility, #Kisspeptin, #NeurokininB
Infertility is a growing health problem that affects millions of people around the world. As a consequence, the use of assisted reproductive technology (ART) is continuously increasing and accounts for approximately 1% to 3% of annual births in developed countries (1). Causes of infertility may vary greatly depending on socioeconomic and geograph ical factors, affecting one or both members of a couple. According to global statistics, 50% of infertility cases are due to female factors, 30% to male factors, and 20% to combined factors. Yet many cases are included within the category of‘‘unexplained infertility’’ to reflect that the cause remains unknown after a complete diagnosis (2, 3). Three major disorder categories contribute to 75% to 80% of those infertility cases that can be explained: disorders of the female tract, ovulation disorders, and poor quality of spermatozoa. The increasing delay in parenthood that characterizes Western societies also impacts greatly the chances of achieving pregnancy (3, 4). If we focus on female infertility, the main indications that lead our patients to seek in vitro fertilization
(IVF) treatment are advanced maternal age, low ovarian response (LOR), polycystic ovarian syndrome (PCOS), and endometriosis.
The rising prevalence and global character of infertility make absolutely necessary the improvement of assisted reproductive treatments and the finding of biomarkers that could serve as diagnostic tools to quickly and accurately assess the current fertility status of a patient. In recent years,
it has been demonstrated that neurokinin B (NKB) and its cognate receptor, NK3R, and kisspeptin (KISS1) and its receptor, KISS1R, play a key role in the regulation of reproduction, and their discovery has allowed a breakthrough in our knowledge of reproductive function (5–12). In humans, kisspeptin and KISS1R are encoded by the KISS1 and the KISS1R
genes, respectively (6, 10, 12). NKB and NK3R belong to the family of tachykinins and are encoded by the TAC3 and TACR3 genes, respectively (13–15). The NKB/NK3R and KISS1/KISS1R systems act primarily at the hypothalamic level of the gonadotropic axis where they modulate gonadotropin-releasing hormone (GnRH) secretion and gonadotropin release (7, 12, 14, 16, 17). In addition, different reports have shown that NKB, NK3R, KISS1, and KISS1R mRNAs or proteins are expressed in peripheral reproductive tissues, particularly in the uterus, the ovary, and the placenta of different mammalian species, including humans (8, 9, 18–26). However, further studies are necessary to increase our knowledge about their role in peripheral tissues and their local effects in the regulation of fertility (5, 7, 27).
Results from other laboratories and ours have shown that NKB, KISS1, and their corresponding receptors are present in human ovarian mural granulosa cells (MGCs) and cumulus cells (CCs) (19, 24–26, 28), and their expression is altered in women with PCOS (23). Nevertheless, little is known about the expression of these systems in infertile women with
other etiologies. In this work, we have analyzed the expression of KISS1, KISS1R, TAC3, and TACR3 in human MGCs and CCs from healthy oocyte donors (as controls) and patients with different infertility diagnoses, including endometriosis, LOR, age-related infertility, PCOS, and unex-
plained infertility, to investigate the expression pattern of these systems in association with the most common causes of women infertility.
MATERIALS AND METHODS
Study population
Approval for this Genetic Association Study was obtained from the institutional ethics committees of CSIC and Hospital Virgen Macarena (Seville, Spain), and all patients gave informed written consent. The study was registered on ClinicalTrials.gov with the code NCT02877992. Human
MGCs and CCs were collected from the preovulatory follicles of Caucasian women, aged 19–45 years, who were undergo ing oocyte retrieval after controlled ovarian stimulation (COS) treatment at the clinic IVI-RMA Seville (IVI-RMA Global) for Reproductive Care. In a first set of experiments, CCs were collected from 162 women divided into two groups: healthy oocyte donors and infertile patients of any etiology, including age-related infertility, endometriosis, PCOS, and unexplained infertility. The
donors group included 52 women, and the infertile group included 110 women: 33 with PCOS, 40 with age-related infertility, 15 with unexplained infertility, and 22 with endometriosis. In a second series of experiments, human MGCs and CCs were collected from 118 women divided into four
groups: 45 were healthy oocyte donors, 27 were women with age-related infertility (R38 years old), 25 had endometriosis, and 21 were low responders. The intention of this division was to detect specific expression profiles for each infertility indication.
A general clinical examination of all patients was performed during the first visit to the fertility practice. Blood samples were obtained during the early follicular phase of their menstrual cycle (day 3) and after administration of the ovulation inductor. Serum hormone levels were assayed enzymatically using an automated biochemistry analyzer (cobas e 411; Roche Diagnostics GmbH).
Eligibility criteria
The healthy oocyte donors group included women between the ages of 18 and 33 years who had functional ovaries and uterus, an antral follicle count (AFC) between 12 and 35, and a normal karyotype. They also underwent a thorough study to exclude mental disorders, hereditary diseases, and common genetic disorders including cystic fibrosis, fragile-
X syndrome and glucose-6-phosphate dehydrogenase (G6PD) deficiency.
The advanced maternal age group included women of age R38 years old with infertility linked primarily to age factor. The endometriosis group included women with infertility associated primarily with endometriosis as diagnosed through transvaginal ultrasound analysis or laparoscopy according to European Society of Human Reproduction and Embryology
(ESHRE) criteria (29). The LOR group included women diagnosed as low responders to COS according to Bologna criteria (30)—that is, presenting two episodes of low response after maximal ovarian stimulation (condition sufficient to define a patient as low responder) or at least two of the following three features: advanced maternal age (R40 years) or any
other risk factor for LOR; a previous LOR (%3 oocytes with
conventional stimulation); and an abnormal ovarian reserve
test (AFC <5–7 and antimullerian hormone [AMH] € <1.1 ng/mL). In the PCOS group, the disease was diagnosed according to 2003 Rotterdam Criteria (31), including any two of the following three clinical features: menstrual dysfunction (oligo/anovulation); clinical and/or biochemical hyperandrogenism; and polycystic ovaries on ultrasound. The unex-
plained infertility group included women with infertility of unknown etiology after a complete infertility evaluation. The eligibility criteria for women of all groups were as follows: body mass index %28 kg/m2, nonsmokers, lack of alcohol consumption, lack of diseases such as hydrosalpinx, congenital adrenal hyperplasia, thyroid disease, Cushing syndrome, androgen-secreting tumors, and lack of use of any drug (medication) that could interfere with ovarian folliculogenesis.
Ovarian Stimulation Protocol
Women were given a standard GnRH-antagonist protocol for COS. We used a combination of two gonadotropins: recombinant follicle-stimulating hormone (FSH) (Gonal F; Merck Serono) and human menopausal gonadotropin (hMG) (Menopur; Ferring Pharmaceuticals). Depending on the AMH level and BMI, the gonadotropin daily doses ranged from 150 IU of recombinant FSH þ 37.5 IU of hMG to 225 IU of recombinant FSH þ 75 IU of hMG. Gonadotropin administration started the second day of the menstrual cycle, after we had checked the ovarian basal status during either the luteal phase of the previous cycle or the first 2 days of
menses, using ultrasound scanning. The GnRH-antagonist (Orgalutran; MSD) was introduced the fifth or sixth day of COS or when the leading follicle had reached a 14-mm diameter. The GnRH-antagonist was administered in a daily dose of 0.25 mg until the day of ovulation induction. Ovulation was induced when at least two follicles had reached a diameter of 17 mm, using 6,500 IU of human chorionic gonado-
tropin (hCG) (Ovitrelle; Merck Serono) or 0.2 mg of the GnRH-agonist triptorelin (Ipsen Pharmabiotech). The latter option was chosen when the risk of ovarian hyperstimulation syndrome had been determined. Gonadotropin doses were adjusted according to patient characteristics and follicular development, which was monitored through periodical ultra-
sound scans and blood estradiol (E2) analysis.
Collection of Human MGCs and CCs
We collected MGCs from the follicular fluids obtained via ultrasound-guided transvaginal oocyte retrieval, which was performed under intravenous anesthesia 36 hours after ovulation induction. After removal of oocyte–cumulus complexes, the remaining follicular aspirates from each patient were pooled and MGCs collected by using the Dynabeads methodology, as described elsewhere (24).
Human CCs were also obtained from these same patients and were collected after procedures described elsewhere (24). After follicular aspiration, the CCs surrounding the oocyte were removed using cutting needles by subsequent treatment of cumulus–oocyte complexes with Sydney IVF Hyaluronidase (80 IU/mL, K-SIHY; Cook Medical) and by carefully removing the CCs of the corona radiata with very thin glass
pipettes (Swemed denudation pipette, 0.134–0.145 mm; Vitrolife).
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from fresh MGCs and CCs using the RNA/Protein purification kit (Norgen Biotek), and residual genomic DNA was removed with RNase-free DNase I and RNasin (Promega). Complementary DNAs (cDNAs) were synthesized using the Transcriptor First Strand cDNA Synthesis kit (Roche). Samples were then preamplified using the SsoAd-
vanced PreAmp supermix (Bio-Rad Laboratories) following the manufacturer’s protocol.
Real-time quantitative polymerase chain reaction (RT-qPCR) was used to quantify the expression of KISS1, KISS1R, TAC3, and TACR3 in CCs and MGCs using the 2DDC T method, as reported elsewhere (24, 32). We performed RT-qPCR on a Bio-Rad iCycler iQ real-time detection apparatus
using SsoAdvanced Universal SYBR Green Supermix (Bio- Rad Laboratories). The parameters of PCR amplification were 10 seconds at 94C, 20 seconds at 60C, and 30 seconds at 72C, for 50 cycles. The sequences of the specific primer pairs designed to amplify each target gene are shown in Supplemental Table 1 (available online). Supplemental
Table 1 also shows the primers used to amplify b-actin (ACTB), hypoxanthine phosphoribosyltransferase 1 (HPRT1), cyclophilin A (PPIA), and succinate dehydrogenase complex subunit A (SDHA), which were chosen as house-keeping genes on the basis of previous studies from other laboratories and ours (24, 33). The specificity of the PCR reactions was confirmed by melting curve analysis of the products and by size verification of the amplicon in a conventional agarose gel. A human universal reference total RNA (BD Biosciences Clontech) was used as a positive control of amplification, and three negative controls were run for each assay: no template, no reverse transcriptase, and no RNA in the reverse transcriptase reaction. Each assay was performed in triplicate,
and the fold change of each target gene expression was expressed relative to the geometric mean mRNA expression of the reference genes in each sample (24, 32).
Statistical Analysis
The results are expressed as mean standard deviation, and n represents the number of experiments in n different women. Data distribution and homogeneity of variances were analyzed with the Kolmogorov-Smirnov test and Levene test. For gene expression data, a logarithmic transformation was adapted to meet the normality assumptions and the statistical differences between these log-transformed values were assessed using Student’s t-test. The relative quantification values are shown in figures without log transformation.
General linear models were performed to control forconfounding variables, and all models were adjusted by BMI and E2 serum levels after ovulation induction. P<.05 was considered statistically significant. All the statistical analyses were performed using IBM SPSS Statistics software, version 24.0.
RESULTS
Expression of KISS1/KISS1R and TAC3/TACR3 in Women with Infertility of any Etiology
We analyzed the expression of the KISS1 and NKB systems in CCs from oocyte donors and infertile women of different etiologies, including the most common disorders with indication of IVF treatment. The anthropometric and biochemical characteristics of healthy donors and infertile patients are shown in Supplemental Table 2 (available online).
Controlled ovarian stimulation for IVF induces a multiple follicular growth that causes great variation in follicular steroids compared with the physiological levels of a natural cycle (see Supplemental Table 2). To avoid any impact of these variations, all women included in the present study, both donors and patients, were given the same treatment, and serum levels of E2 and progesterone (P4) were measured after administration of the ovulation inductor.
In our study, there was a statistically significant variation in E2 serum levels in infertile patients (P<.0001, n 1⁄4 110) with respect to healthy donors (n 1⁄4 52) (Supplemental Table 2) and no variation in P4 serum levels (P>.05) (Supplemental Table 2). There were statistically significant
differences between fertile and infertile women in age and BMI (Supplemental Table 2). The serum hormone levels and the expression levels of all the genes examined were not influenced by the use of recombinant hCG or triptorelin for ovulation induction.
The expression of KISS1 was down-regulated in CCs from infertile patients, in comparison with mRNA levels in control healthy women (Supplemental Table 2). The differences remained statistically significant when adjusted for BMI and serum levels of E2 after ovulation induction (b 1⁄4 0.303, P1⁄4.001). Conversely, no statistically significant differences were observed in relation to the KISS1R expression when comparing both groups (Supplemental Table 2). The expression of TAC3 was lower in CCs from infertile patients (Supplemental Table 2), and these differences re-
mained statistically significant after adjusting for BMI and serum E2 after ovulation induction (b 1⁄4 0.259, P1⁄4.008). A multiple linear regression analysis shows that infertility was also associated with a lower expression of TACR3 mRNA in CCs, which remained statistically significant after
adjusting for BMI and E2 serum levels after ovulation induction (b 1⁄4 0.335, P1⁄4.001) (Supplemental Table 2).
Clinical Characteristics of Healthy Donors and Women with Age-Related Infertility, Endometriosis, and Low Ovarian Response
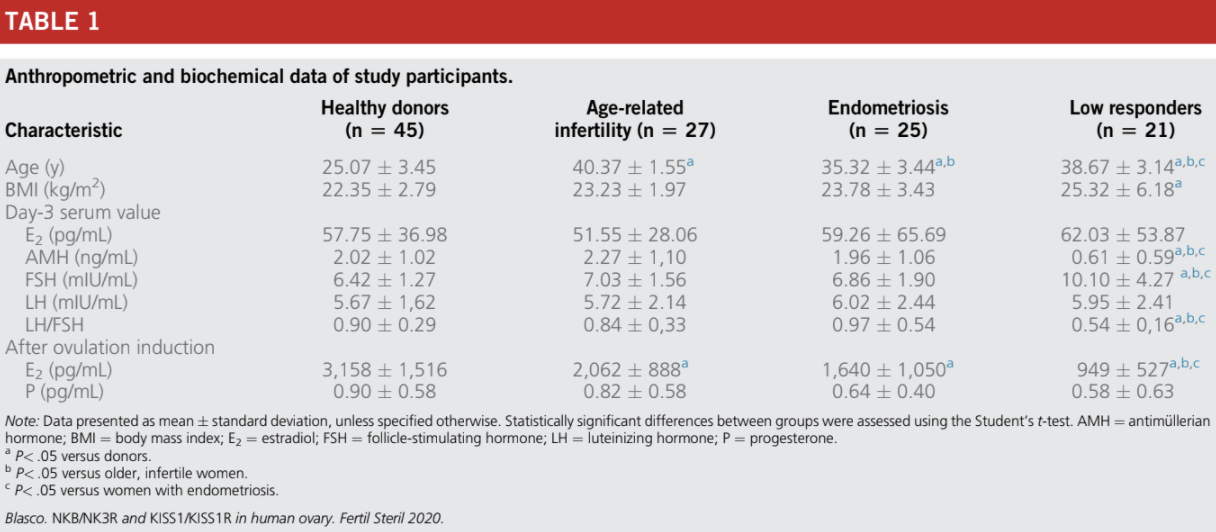
The biochemical and anthropometric parameters of the women included in the study are shown in Table 1. The serum concentrations of E2, AMH, FSH, and luteinizing hormone (LH) fell within the reference range values in the early follicular phase of the menstrual cycle in healthy donors and in
women with infertility due to age (R38 years old), endometriosis, and LOR (Table 1). The serum concentrations of day 3 E2, day-3 LH, and P4 measured after administration of the ovulation inductor were similar in the control and infertile groups. There were statistically significant differences between the groups in relation to the other parameters analyzed (Table 1).
Expression of KISS1/KISS1R and TAC3/TACR3 in Women with Age-Related Infertility
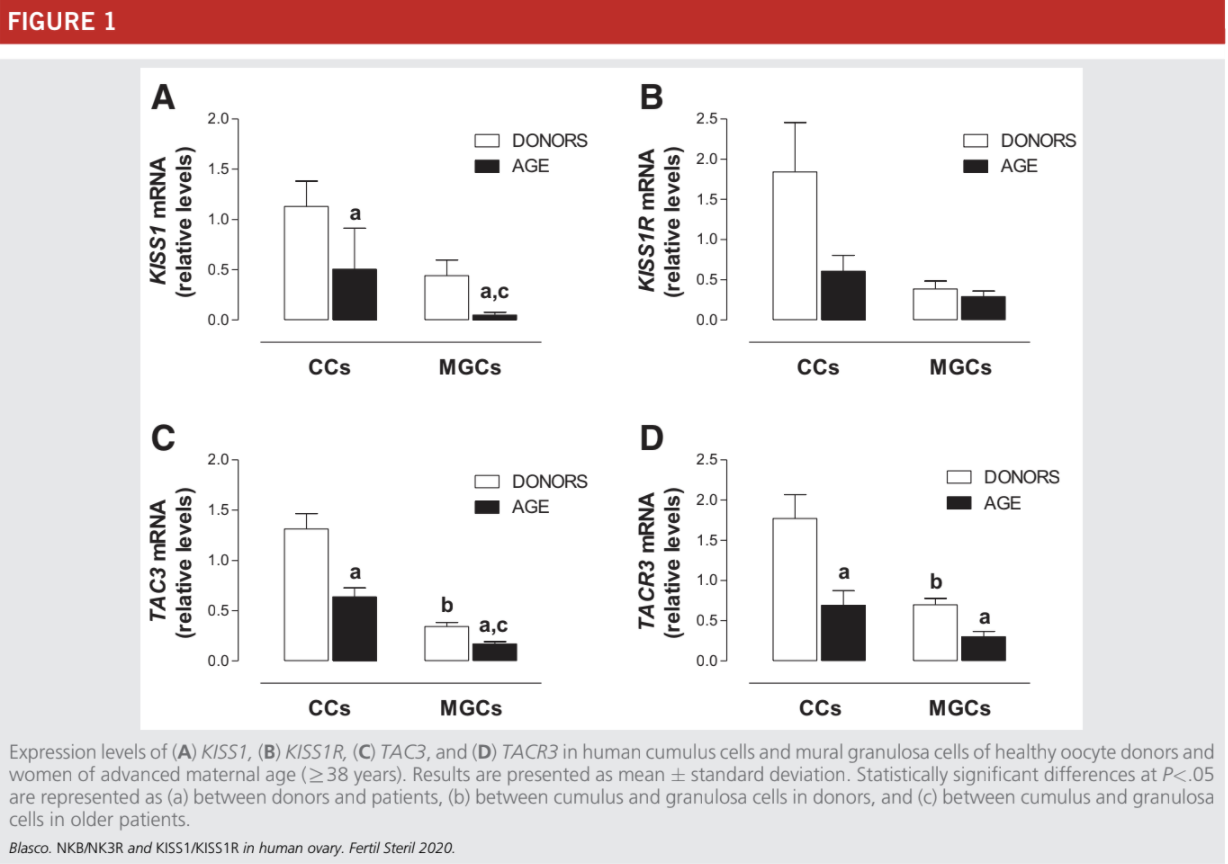
The expression of KISS1, TAC3, and TACR3 was statistically significantly lower in CCs and MGCs of the older women (R38 years) in comparison with mRNA levels in the control healthy women (Fig. 1A, C, and D). The expression levels of KISS1R were lower in older women but showed great variations between samples; as a consequence, the differences
between older infertile patients and control women did not reach statistical significance (Fig. 1B).
In agreement with our previous data (23, 24), the expression of TAC3 and TACR3 were higher in CCs than in MGCs from healthy donors (Fig. 1C and D). In older infertile patients there was also a statistically significant increase in the expression of TAC3 mRNA in CCs, in comparison with
MGCs, but this increase was not observed for TACR3 (Fig 1D).
Expression of KISS1/KISS1R and TAC3/TACR3 in Women with Endometriosis
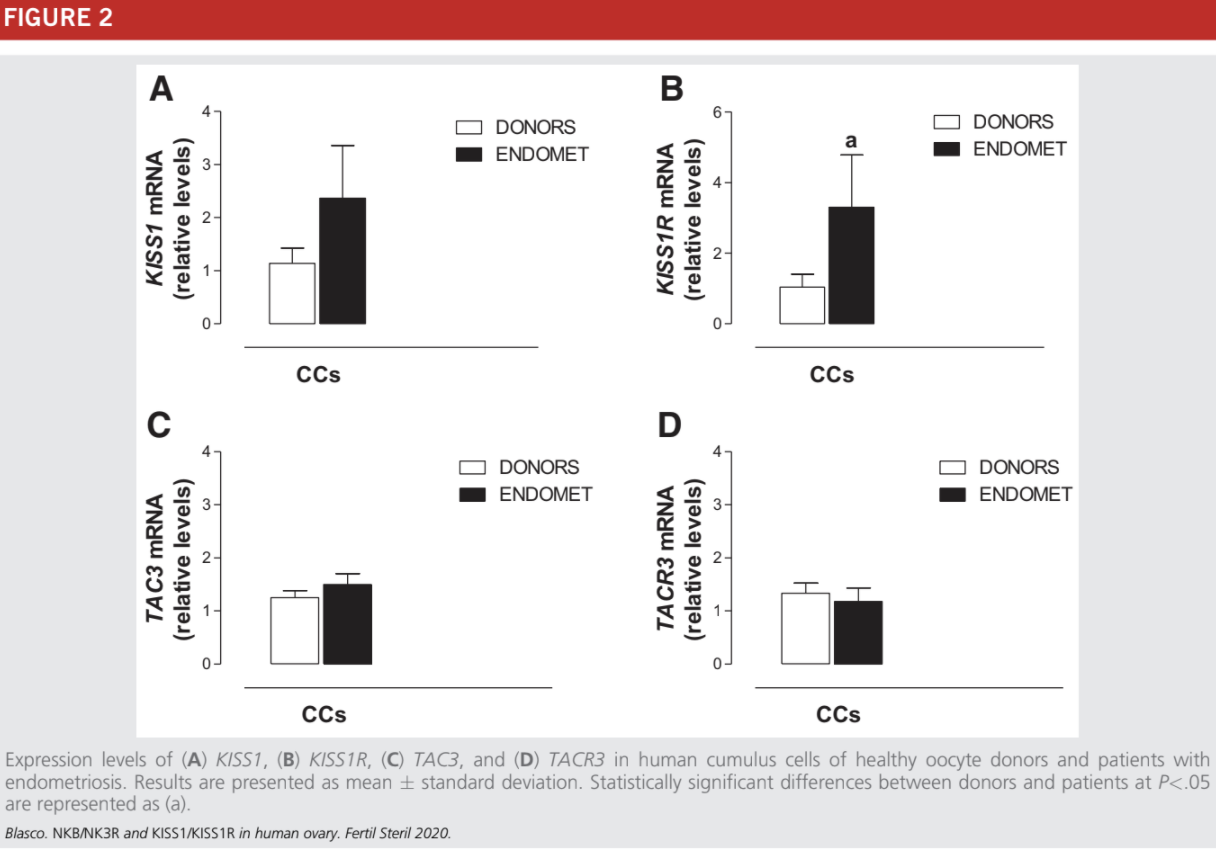
This study was performed only in CCs to avoid the analysis of damaged MGCs from women with endometriosis. The expression levels of KISS1, TAC3, and TACR3 were similar in infertile women with endometriosis and healthy women (Fig. 2A, C, and D). The expression of KISS1R was statistically significantly higher in CCs from women with endometriosis
(Fig. 2B).
Within this group, there were nine patients R38 years old. In four of these women, the expression levels of TAC3/TACR3 and KISS1/KISS1R were comparable with those observed in the group of older infertile women: they showed a lower expression of these genes in comparison with healthy
control women. A decreased expression of TAC3, TACR3, and KISS1 was also observed in five patients with endometriosis who were %38 years old.
Expression of KISS1/KISS1R and TAC3/TACR3 in Low-Responder Women
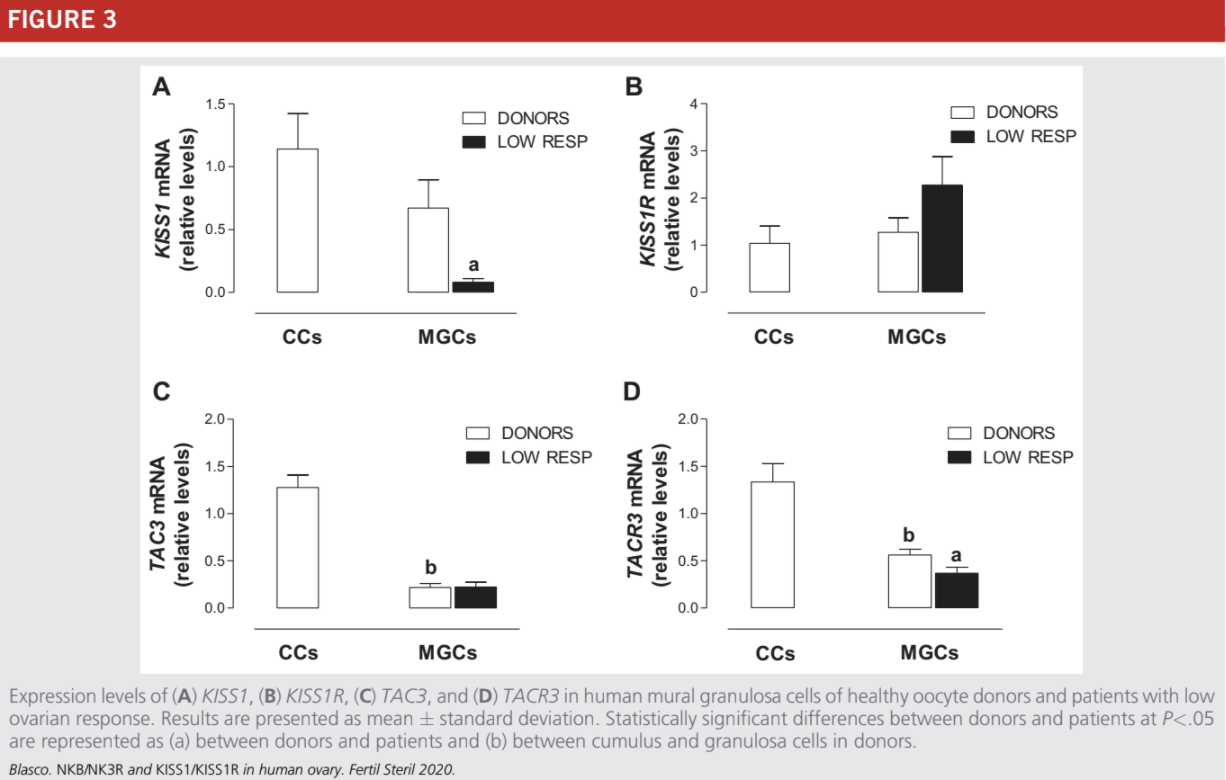
Due to the small quantity of CCs obtained from low-responder patients, this study was performed only on MGCs to use the same PCR experimental conditions with all samples analyzed. As occurs in MGCs from age-related infertile women (Fig. 1) and PCOS patients (23), the TAC3 and KISS1R mRNA levels were not statistically significantly different between the con-
trols and low responders (Fig. 3B and C) whereas the expression levels of KISS1 and TACR3 were statistically significantly lower in MGCs from infertile women with low response in comparison with healthy women (Fig 3A and D).
DISCUSSION
Neurokinin B and its receptor NK3R together with KISS1 and its receptor KISS1R exert an essential role in the brain as regulators of the hypothalamic-pituitary-gonadal axis. This discovery has contributed to an unprecedented advance in our knowledge about reproductive function regulation (11, 12, 34, 35). Moreover, experimental data gathered in
recent years prove that these systems are also expressed in the female genital tract (endometrium, oviduct, and ovary), suggesting that they act as important local regulators of reproductive function (8, 9, 18–20, 22, 28). Recent data also suggest that KISS1 signaling is necessary for a correct embryo implantation and placentation (21). The main finding of this study is that expression of the NKB/NK3R and KISS1/KISS1R systems is altered in granulosa cells from infertile women with different infertility etiologies as compared with healthy oocyte donors. These results confirm that these systems are indeed important for correct ovarian function and fertility. We have observed that NKB (encoded by TAC3), NK3R (encoded by TACR3), and KISS1 (encoded by KISS1) expression is statistically significantly down-regulated in the cumulus cells of infertile patients
considered as a whole group, including with the most frequent disorders in women attending an IVF treatment: advanced maternal age, PCOS, endometriosis, LOR, and unexplained infertility. These results suggest that altered expression of these genes might be responsible, at least in part, for the infertility experienced by the patients.
In previous studies, we found that NKB/NK3R andvKISS1/KISS1R systems are present in human MGCs and CCs (19, 23–25). Moreover, we compared the expression of these systems between oocyte donors and infertile women with PCOS, and we found statistically significant differences (23). TAC3, TACR3, and KISS1 mRNAs were down-regulated in MGCs and CCs from PCOS patients, which led us to wonder whether these results would repeat in patients with other infertility etiologies. In fact, in the present study we observed similar results in the infertile patients group, the one including all etiologies. This makes sense because oocyte quality is affected in all these etiologies. Different ovarian stimulation programs did not modify or were unable to induce a recovery of the expression of the
TAC3/TACR3 and KISS1/KISS1R systems to the levels observed in healthy donors. All in all, an expression analysis of the NKB and KISS1 systems in MGCs or CCs would thus be useful to assess the fertility status of a patient. In the case of PCOS, it is very probable that other genes are affected and their expression levels vary depending on how PCOS is man-
ifested in the patient because it is a very heterogeneous disease. New knowledge on the genetic alterations behind this syndrome could allow us to stablish a better, more specific genetic profile for PCOS.
Each cause of infertility displayed different anthropometric and hormone data, allowing a specific profile for each type of disease. Controlled ovarian stimulation for IVF induces multiple follicular growth, which causes a dramatic variation of ovarian steroids E2 and P4 in comparison with
the physiologic levels of a natural menstrual cycle. According to our results, the observed dysregulation of the NKB/NK3R and KISS1/KISS1R systems cannot be attributed to differences in the hormone state. Instead, it seems to be directly related to the infertility status (Table 1 and Supplemental Table 2).
Two fertility trends of the 21st century have become evident in the Western countries: women are having fewer children, and they are delaying births to a later age. Furthermore, women who choose to delay motherhood may encounter delays and/or disappointment due to decreased fecundity (36). Age-related infertility comprises several causes leading to infertility. On the one hand, there is a reduction in the number of oocytes (ovarian reserve) as age increases. On the other hand, oocyte quality is also impaired with advancing age due to a widely described correlation between female age and oocyte chromosomal abnormalities,
leading to a higher rate of miscarriage and genetic disorders in the fetus. This higher frequency of aneuploidies is due to alterations in the regulatory machinery responsible for assembly of the oocyte meiotic spindle.
Furthermore, aging is also associated with the appearance of other infertility-related disorders such as tubal disease, leiomyomas, and endometriosis (37). In this study, the group of women of advanced maternal age showed a statistically significant down-regulation in KISS1, TAC3, and TACR3 levels, suggesting that altered expression of these genes might be involved in the impaired oocyte quality and/or the decreased ovarian reserve associated with advancing age. In fact, previous studies performed in different animal models have already found an association between kisspeptin and follicle development and oocyte maturation (18, 20, 26, 38). Endometriosis is a chronic inflammatory disease that cause pain and infertility in women, with a prevalence of 0.8% to 6.0% in population-based studies (39–41) and 20% to 50% in subfertile women (42, 43). Endometriosis is characterized by the growing of endometrial-like tissue in ectopic locations such as the oviduct, ovary, or peritoneal cavity. The origin and pathogenesis of this disease remains unclear, and different theories have been postulated to explain this phenomenon. Some theories propose that endometrial implants come from uterine endometrium, and other theories propose that these implants arise from other tissues, involving a process of transformation (44). The abnormally implanted tissue responds cyclically to hormones, developing inflammatory responses. Consequently, patients may develop pelvic adhesions and experience pain and infertility (45). Endometriosis has been related to impaired oocyte quality, reduced fertility, and lower implantation rates after IVF, but the link between infertility and endometriosis is still poorly
understood (46). Genetic and epigenetic changes have been associated with endometriosis, which is considered a hereditary disease, and many cases have been attributed to hereditary factors (47).
A recent study has detected that KISS1 expression is statistically significantly higher in endometriosis lesions in comparison with eutopic glandular endometrium, suggesting a possible role of KISS1 in endometriosis pathogenesis (48).
However, a different study did not detect KISS1 expression in any sample from endometriosis patients (49), which could be due to methodological or design differences between the studies. In our case, we analyzed granulosa cells from endometriosis patients and found that they constituted a very heterogeneous group in relation to the expression of the TAC3/ TACR3 and KISS1/KISS1R systems; when considered as a whole, only the expression of KISS1R was altered. KISS1R mRNA levels were statistically significantly higher in CCs from endometriosis patients in comparison with healthy oocyte donors. Thus, the increased expression of KISS1R could be one of the multiple factors involved in the origin of endometriosis and related infertility. These data suggest that endometriosis is a very different entity in comparison with advanced maternal age and LOR as a cause of infertility. As mentioned earlier, there is still much to know about the origin and causes of this disease.
Further studies are needed to clarify the reasons behind the increase in KISS1R expression and investigate the potential relationship between KISS1/KISS1R and endometriosis. If confirmed, KISS1 and/or its receptor could serve as biomarkers for endometriosis diagnosis and detection.
Low ovarian response indicates a reduction in the number of oocytes retrieved after an ovarian puncture due to a diminished follicular response to COS (30, 50). The existence of LOR was unveiled thanks to the increasing acceptance and spreading of ART. Approximately 10% of women undergoing IVF treatment will show LOR to COS. However, this incidence can be higher in the infertile population, as many affected
women never undergo an ART treatment (51). Regarding the results of our study, lower expression levels of KISS1 and TACR3 were observed in the MGCs of women with LOR in comparison with healthy oocyte donors.
This altered expression profile suggests the possible involvement of these factors in the correct follicle recruitment and development in response to gonadotropin stimulation. These results are concordant with previous studies that have identified the involvement of KISS1/KISS1R system in the regulation of follicular development, oocyte maturation, ovulation,
and ovarian steroidogenesis (28). Our results suggest that, besides kisspeptin expression levels, correct expression levels of NKB might also be necessary for normal folliculogenesis. In relation to advanced-age patients and those with LOR, it is worth pointing to a previous study performed in mice that revealed that a defect in the KISS1/KISS1R system induced a state similar to premature ovarian failure. Mutant mice
showed a premature decline in ovulatory rate, progressive loss of oocytes and antral follicles, and reduced fertility. This is concordant with results of our study because both older patients and low responders showed decreased levels of KISS1 in comparison with healthy oocyte donors (20).
CONCLUSION
Our study has revealed a differential and altered regulation of the NKB/NK3R and KISS1/KISS1R systems in cumulus and granulosa cells from women with infertility of different etiologies, particularly in patients with advanced age, endometriosis, and LOR. We provide evidence that an abnormal expression of these systems at the ovarian level might be
involved in the decreased fertility of these patients expression of these systems at the ovarian level might be involved in the decreased fertility of these patients.
REFERENCES
1. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–26.
2. Agarwal A, Majzoub A, Parekh N, Henkel R. A schematic overview of the
current status of male infertility practice. World J Mens Health 2019;37:e41.
3. Johnson MH. Essential reproduction. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2018.
4. Healy DL, Trounson AO, Andersen AN. Female infertility: causes and treatment. Lancet 1994;343:1539–44.
5. Candenas L, Lecci A, Pinto FM, Patak E, Maggi CA, Pennefather JN. Tachykinins and tachykinin receptors: effects in the genitourinary tract. Life Sci 2005;76:835–62.
6. De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003;100:10972 6.
7. Lehman MN, Coolen LM, Steiner RA, Neal-Perry G, Wang L, Moenter SM,
et al. The 3rd World Conference on Kisspeptin, ‘‘Kisspeptin 2017: Brain
and Beyond’’: Unresolved questions, challenges and future directions for
the field. J Neuroendocrinol 2018;30:e12600.
8. Page NM, Woods RJ, Gardiner SM, Lomthaisong K, Gladwell RT, Butlin DJ,et al. Excessive placental secretion of neurokinin B during the third trimester causes pre-eclampsia. Nature 2000;405:797–800.
9. Pinto FM, Armesto CP, Magraner J, Trujillo M, Martin JD, Candenas ML. Tachykinin receptor and neutral endopeptidase gene expression in the rat uterus: characterization and regulation in response to ovarian steroid treatment. Endocrinology 1999;140:2526–32.
10. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr,
Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J
Med 2003;349:1614–27.
11. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al.TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism
reveal a key role for Neurokinin B in the central control of reproduction.
Nat Genet 2009;41:354–8.
12. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev2012;92:1235–316.
13. Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 2004;11:2045–81.
14. Hu G, Lin C, He M, Wong AO. Neurokinin B and reproductive functions:
‘‘KNDy neuron’’ model in mammals and the emerging story in fish. Gen
Comp Endocrinol 2014;208:94–108.
15. Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets 2006;7:963–74.
16. Clarke H, Dhillo WS, Jayasena CN. Comprehensive Review on Kisspeptin and Its Role in Reproductive Disorders. Endocrinol Metab (Seoul) 2015;30:124–41.
17. Skorupskaite K, George JT, Veldhuis JD, Anderson RA. Neurokinin B Regulates Gonadotropin Secretion, Ovarian Follicle Growth, and the Timing of Ovulation in Healthy Women. J Clin Endocrinol Metab 2018;103:95–104.
18. Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology 2006;147:4852–62.
19. Cejudo Roman A, Pinto FM, Dorta I, Almeida TA, Hernandez M, Illanes M,et al. Analysis of the expression of neurokinin B, kisspeptin, and their
cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril 2012;97:1213–9.
20. Gaytan F, Garcia-Galiano D, Dorfman MD, Manfredi-Lozano M,
Castellano JM, Dissen GA, et al. Kisspeptin receptor haplo-insufficiency
causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology 2014;155:3088–97.
To analyze and compare the expression profile of TAC3, TACR3, KISS1, and KISS1R in mural granulosa and cumulus cells
from healthy oocyte donors and patients with different infertility etiologies, including advanced maternal age, endometriosis, and low
ovarian response.
Design: Genetic association study.
Setting: Private fertility clinic and public research laboratory.
Patient(s): Healthy oocyte donors and infertile women undergoing in vitro fertilization (IVF) treatment.
Intervention(s): IVF.
Main Outcome Measure(s): Gene expression levels of KISS1, KISS1R, TAC3, and TACR3 in human mural granulosa and cumulus
cells.
Result(s): Infertile women showed statistically significantly altered expression levels of KISS1 (2.57 2.30 vs. 1.37 2.11), TAC3
(1.21 1.40 vs. 1.49 1.98), and TACR3 (0.77 1.36 vs. 0.03 0.56) when compared with healthy oocyte donors. Advanced
maternal age patients, endometriosis patients, and low responders showed specific and altered expression profiles in comparison with
oocyte donors.
Conclusion(s): Abnormal expression levels of KISS1/KISS1R and TAC3/TACR3 systems in granulosa cells might be involved in the
decreased fertility associated to advanced maternal age, endometriosis, and low ovarian response. (Fertil Steril 2020;114:869-78.
2020 by American Society for Reproductive Medicine.)
El resumen está disponible en Español al final del artículo.
Key Words: #GranulosaCells, #humanInfertility, #Kisspeptin, #NeurokininB
Infertility is a growing health problem that affects millions of people around the world. As a consequence, the use of assisted reproductive technology (ART) is continuously increasing and accounts for approximately 1% to 3% of annual births in developed countries (1). Causes of infertility may vary greatly depending on socioeconomic and geograph ical factors, affecting one or both members of a couple. According to global statistics, 50% of infertility cases are due to female factors, 30% to male factors, and 20% to combined factors. Yet many cases are included within the category of‘‘unexplained infertility’’ to reflect that the cause remains unknown after a complete diagnosis (2, 3). Three major disorder categories contribute to 75% to 80% of those infertility cases that can be explained: disorders of the female tract, ovulation disorders, and poor quality of spermatozoa. The increasing delay in parenthood that characterizes Western societies also impacts greatly the chances of achieving pregnancy (3, 4). If we focus on female infertility, the main indications that lead our patients to seek in vitro fertilization
(IVF) treatment are advanced maternal age, low ovarian response (LOR), polycystic ovarian syndrome (PCOS), and endometriosis.
The rising prevalence and global character of infertility make absolutely necessary the improvement of assisted reproductive treatments and the finding of biomarkers that could serve as diagnostic tools to quickly and accurately assess the current fertility status of a patient. In recent years,
it has been demonstrated that neurokinin B (NKB) and its cognate receptor, NK3R, and kisspeptin (KISS1) and its receptor, KISS1R, play a key role in the regulation of reproduction, and their discovery has allowed a breakthrough in our knowledge of reproductive function (5–12). In humans, kisspeptin and KISS1R are encoded by the KISS1 and the KISS1R
genes, respectively (6, 10, 12). NKB and NK3R belong to the family of tachykinins and are encoded by the TAC3 and TACR3 genes, respectively (13–15). The NKB/NK3R and KISS1/KISS1R systems act primarily at the hypothalamic level of the gonadotropic axis where they modulate gonadotropin-releasing hormone (GnRH) secretion and gonadotropin release (7, 12, 14, 16, 17). In addition, different reports have shown that NKB, NK3R, KISS1, and KISS1R mRNAs or proteins are expressed in peripheral reproductive tissues, particularly in the uterus, the ovary, and the placenta of different mammalian species, including humans (8, 9, 18–26). However, further studies are necessary to increase our knowledge about their role in peripheral tissues and their local effects in the regulation of fertility (5, 7, 27).
Results from other laboratories and ours have shown that NKB, KISS1, and their corresponding receptors are present in human ovarian mural granulosa cells (MGCs) and cumulus cells (CCs) (19, 24–26, 28), and their expression is altered in women with PCOS (23). Nevertheless, little is known about the expression of these systems in infertile women with
other etiologies. In this work, we have analyzed the expression of KISS1, KISS1R, TAC3, and TACR3 in human MGCs and CCs from healthy oocyte donors (as controls) and patients with different infertility diagnoses, including endometriosis, LOR, age-related infertility, PCOS, and unex-
plained infertility, to investigate the expression pattern of these systems in association with the most common causes of women infertility.
MATERIALS AND METHODS
Study population
Approval for this Genetic Association Study was obtained from the institutional ethics committees of CSIC and Hospital Virgen Macarena (Seville, Spain), and all patients gave informed written consent. The study was registered on ClinicalTrials.gov with the code NCT02877992. Human
MGCs and CCs were collected from the preovulatory follicles of Caucasian women, aged 19–45 years, who were undergo ing oocyte retrieval after controlled ovarian stimulation (COS) treatment at the clinic IVI-RMA Seville (IVI-RMA Global) for Reproductive Care. In a first set of experiments, CCs were collected from 162 women divided into two groups: healthy oocyte donors and infertile patients of any etiology, including age-related infertility, endometriosis, PCOS, and unexplained infertility. The
donors group included 52 women, and the infertile group included 110 women: 33 with PCOS, 40 with age-related infertility, 15 with unexplained infertility, and 22 with endometriosis. In a second series of experiments, human MGCs and CCs were collected from 118 women divided into four
groups: 45 were healthy oocyte donors, 27 were women with age-related infertility (R38 years old), 25 had endometriosis, and 21 were low responders. The intention of this division was to detect specific expression profiles for each infertility indication.
A general clinical examination of all patients was performed during the first visit to the fertility practice. Blood samples were obtained during the early follicular phase of their menstrual cycle (day 3) and after administration of the ovulation inductor. Serum hormone levels were assayed enzymatically using an automated biochemistry analyzer (cobas e 411; Roche Diagnostics GmbH).
Eligibility criteria
The healthy oocyte donors group included women between the ages of 18 and 33 years who had functional ovaries and uterus, an antral follicle count (AFC) between 12 and 35, and a normal karyotype. They also underwent a thorough study to exclude mental disorders, hereditary diseases, and common genetic disorders including cystic fibrosis, fragile-
X syndrome and glucose-6-phosphate dehydrogenase (G6PD) deficiency.
The advanced maternal age group included women of age R38 years old with infertility linked primarily to age factor. The endometriosis group included women with infertility associated primarily with endometriosis as diagnosed through transvaginal ultrasound analysis or laparoscopy according to European Society of Human Reproduction and Embryology
(ESHRE) criteria (29). The LOR group included women diagnosed as low responders to COS according to Bologna criteria (30)—that is, presenting two episodes of low response after maximal ovarian stimulation (condition sufficient to define a patient as low responder) or at least two of the following three features: advanced maternal age (R40 years) or any
other risk factor for LOR; a previous LOR (%3 oocytes with
conventional stimulation); and an abnormal ovarian reserve
test (AFC <5–7 and antimullerian hormone [AMH] € <1.1 ng/mL). In the PCOS group, the disease was diagnosed according to 2003 Rotterdam Criteria (31), including any two of the following three clinical features: menstrual dysfunction (oligo/anovulation); clinical and/or biochemical hyperandrogenism; and polycystic ovaries on ultrasound. The unex-
plained infertility group included women with infertility of unknown etiology after a complete infertility evaluation. The eligibility criteria for women of all groups were as follows: body mass index %28 kg/m2, nonsmokers, lack of alcohol consumption, lack of diseases such as hydrosalpinx, congenital adrenal hyperplasia, thyroid disease, Cushing syndrome, androgen-secreting tumors, and lack of use of any drug (medication) that could interfere with ovarian folliculogenesis.
Ovarian Stimulation Protocol
Women were given a standard GnRH-antagonist protocol for COS. We used a combination of two gonadotropins: recombinant follicle-stimulating hormone (FSH) (Gonal F; Merck Serono) and human menopausal gonadotropin (hMG) (Menopur; Ferring Pharmaceuticals). Depending on the AMH level and BMI, the gonadotropin daily doses ranged from 150 IU of recombinant FSH þ 37.5 IU of hMG to 225 IU of recombinant FSH þ 75 IU of hMG. Gonadotropin administration started the second day of the menstrual cycle, after we had checked the ovarian basal status during either the luteal phase of the previous cycle or the first 2 days of
menses, using ultrasound scanning. The GnRH-antagonist (Orgalutran; MSD) was introduced the fifth or sixth day of COS or when the leading follicle had reached a 14-mm diameter. The GnRH-antagonist was administered in a daily dose of 0.25 mg until the day of ovulation induction. Ovulation was induced when at least two follicles had reached a diameter of 17 mm, using 6,500 IU of human chorionic gonado-
tropin (hCG) (Ovitrelle; Merck Serono) or 0.2 mg of the GnRH-agonist triptorelin (Ipsen Pharmabiotech). The latter option was chosen when the risk of ovarian hyperstimulation syndrome had been determined. Gonadotropin doses were adjusted according to patient characteristics and follicular development, which was monitored through periodical ultra-
sound scans and blood estradiol (E2) analysis.
Collection of Human MGCs and CCs
We collected MGCs from the follicular fluids obtained via ultrasound-guided transvaginal oocyte retrieval, which was performed under intravenous anesthesia 36 hours after ovulation induction. After removal of oocyte–cumulus complexes, the remaining follicular aspirates from each patient were pooled and MGCs collected by using the Dynabeads methodology, as described elsewhere (24).
Human CCs were also obtained from these same patients and were collected after procedures described elsewhere (24). After follicular aspiration, the CCs surrounding the oocyte were removed using cutting needles by subsequent treatment of cumulus–oocyte complexes with Sydney IVF Hyaluronidase (80 IU/mL, K-SIHY; Cook Medical) and by carefully removing the CCs of the corona radiata with very thin glass
pipettes (Swemed denudation pipette, 0.134–0.145 mm; Vitrolife).
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from fresh MGCs and CCs using the RNA/Protein purification kit (Norgen Biotek), and residual genomic DNA was removed with RNase-free DNase I and RNasin (Promega). Complementary DNAs (cDNAs) were synthesized using the Transcriptor First Strand cDNA Synthesis kit (Roche). Samples were then preamplified using the SsoAd-
vanced PreAmp supermix (Bio-Rad Laboratories) following the manufacturer’s protocol.
Real-time quantitative polymerase chain reaction (RT-qPCR) was used to quantify the expression of KISS1, KISS1R, TAC3, and TACR3 in CCs and MGCs using the 2DDC T method, as reported elsewhere (24, 32). We performed RT-qPCR on a Bio-Rad iCycler iQ real-time detection apparatus
using SsoAdvanced Universal SYBR Green Supermix (Bio- Rad Laboratories). The parameters of PCR amplification were 10 seconds at 94C, 20 seconds at 60C, and 30 seconds at 72C, for 50 cycles. The sequences of the specific primer pairs designed to amplify each target gene are shown in Supplemental Table 1 (available online). Supplemental
Table 1 also shows the primers used to amplify b-actin (ACTB), hypoxanthine phosphoribosyltransferase 1 (HPRT1), cyclophilin A (PPIA), and succinate dehydrogenase complex subunit A (SDHA), which were chosen as house-keeping genes on the basis of previous studies from other laboratories and ours (24, 33). The specificity of the PCR reactions was confirmed by melting curve analysis of the products and by size verification of the amplicon in a conventional agarose gel. A human universal reference total RNA (BD Biosciences Clontech) was used as a positive control of amplification, and three negative controls were run for each assay: no template, no reverse transcriptase, and no RNA in the reverse transcriptase reaction. Each assay was performed in triplicate,
and the fold change of each target gene expression was expressed relative to the geometric mean mRNA expression of the reference genes in each sample (24, 32).
Statistical Analysis
The results are expressed as mean standard deviation, and n represents the number of experiments in n different women. Data distribution and homogeneity of variances were analyzed with the Kolmogorov-Smirnov test and Levene test. For gene expression data, a logarithmic transformation was adapted to meet the normality assumptions and the statistical differences between these log-transformed values were assessed using Student’s t-test. The relative quantification values are shown in figures without log transformation.
General linear models were performed to control forconfounding variables, and all models were adjusted by BMI and E2 serum levels after ovulation induction. P<.05 was considered statistically significant. All the statistical analyses were performed using IBM SPSS Statistics software, version 24.0.
RESULTS
Expression of KISS1/KISS1R and TAC3/TACR3 in Women with Infertility of any Etiology
We analyzed the expression of the KISS1 and NKB systems in CCs from oocyte donors and infertile women of different etiologies, including the most common disorders with indication of IVF treatment. The anthropometric and biochemical characteristics of healthy donors and infertile patients are shown in Supplemental Table 2 (available online).
Controlled ovarian stimulation for IVF induces a multiple follicular growth that causes great variation in follicular steroids compared with the physiological levels of a natural cycle (see Supplemental Table 2). To avoid any impact of these variations, all women included in the present study, both donors and patients, were given the same treatment, and serum levels of E2 and progesterone (P4) were measured after administration of the ovulation inductor.
In our study, there was a statistically significant variation in E2 serum levels in infertile patients (P<.0001, n 1⁄4 110) with respect to healthy donors (n 1⁄4 52) (Supplemental Table 2) and no variation in P4 serum levels (P>.05) (Supplemental Table 2). There were statistically significant
differences between fertile and infertile women in age and BMI (Supplemental Table 2). The serum hormone levels and the expression levels of all the genes examined were not influenced by the use of recombinant hCG or triptorelin for ovulation induction.
The expression of KISS1 was down-regulated in CCs from infertile patients, in comparison with mRNA levels in control healthy women (Supplemental Table 2). The differences remained statistically significant when adjusted for BMI and serum levels of E2 after ovulation induction (b 1⁄4 0.303, P1⁄4.001). Conversely, no statistically significant differences were observed in relation to the KISS1R expression when comparing both groups (Supplemental Table 2). The expression of TAC3 was lower in CCs from infertile patients (Supplemental Table 2), and these differences re-
mained statistically significant after adjusting for BMI and serum E2 after ovulation induction (b 1⁄4 0.259, P1⁄4.008). A multiple linear regression analysis shows that infertility was also associated with a lower expression of TACR3 mRNA in CCs, which remained statistically significant after
adjusting for BMI and E2 serum levels after ovulation induction (b 1⁄4 0.335, P1⁄4.001) (Supplemental Table 2).
Clinical Characteristics of Healthy Donors and Women with Age-Related Infertility, Endometriosis, and Low Ovarian Response
The biochemical and anthropometric parameters of the women included in the study are shown in Table 1. The serum concentrations of E2, AMH, FSH, and luteinizing hormone (LH) fell within the reference range values in the early follicular phase of the menstrual cycle in healthy donors and in
women with infertility due to age (R38 years old), endometriosis, and LOR (Table 1). The serum concentrations of day 3 E2, day-3 LH, and P4 measured after administration of the ovulation inductor were similar in the control and infertile groups. There were statistically significant differences between the groups in relation to the other parameters analyzed (Table 1).
Expression of KISS1/KISS1R and TAC3/TACR3 in Women with Age-Related Infertility
The expression of KISS1, TAC3, and TACR3 was statistically significantly lower in CCs and MGCs of the older women (R38 years) in comparison with mRNA levels in the control healthy women (Fig. 1A, C, and D). The expression levels of KISS1R were lower in older women but showed great variations between samples; as a consequence, the differences
between older infertile patients and control women did not reach statistical significance (Fig. 1B).
In agreement with our previous data (23, 24), the expression of TAC3 and TACR3 were higher in CCs than in MGCs from healthy donors (Fig. 1C and D). In older infertile patients there was also a statistically significant increase in the expression of TAC3 mRNA in CCs, in comparison with
MGCs, but this increase was not observed for TACR3 (Fig 1D).


Expression of KISS1/KISS1R and TAC3/TACR3 in Women with Endometriosis
This study was performed only in CCs to avoid the analysis of damaged MGCs from women with endometriosis. The expression levels of KISS1, TAC3, and TACR3 were similar in infertile women with endometriosis and healthy women (Fig. 2A, C, and D). The expression of KISS1R was statistically significantly higher in CCs from women with endometriosis
(Fig. 2B).
Within this group, there were nine patients R38 years old. In four of these women, the expression levels of TAC3/TACR3 and KISS1/KISS1R were comparable with those observed in the group of older infertile women: they showed a lower expression of these genes in comparison with healthy
control women. A decreased expression of TAC3, TACR3, and KISS1 was also observed in five patients with endometriosis who were %38 years old.
Expression of KISS1/KISS1R and TAC3/TACR3 in Low-Responder Women
Due to the small quantity of CCs obtained from low-responder patients, this study was performed only on MGCs to use the same PCR experimental conditions with all samples analyzed. As occurs in MGCs from age-related infertile women (Fig. 1) and PCOS patients (23), the TAC3 and KISS1R mRNA levels were not statistically significantly different between the con-
trols and low responders (Fig. 3B and C) whereas the expression levels of KISS1 and TACR3 were statistically significantly lower in MGCs from infertile women with low response in comparison with healthy women (Fig 3A and D).
DISCUSSION
Neurokinin B and its receptor NK3R together with KISS1 and its receptor KISS1R exert an essential role in the brain as regulators of the hypothalamic-pituitary-gonadal axis. This discovery has contributed to an unprecedented advance in our knowledge about reproductive function regulation (11, 12, 34, 35). Moreover, experimental data gathered in
recent years prove that these systems are also expressed in the female genital tract (endometrium, oviduct, and ovary), suggesting that they act as important local regulators of reproductive function (8, 9, 18–20, 22, 28). Recent data also suggest that KISS1 signaling is necessary for a correct embryo implantation and placentation (21). The main finding of this study is that expression of the NKB/NK3R and KISS1/KISS1R systems is altered in granulosa cells from infertile women with different infertility etiologies as compared with healthy oocyte donors. These results confirm that these systems are indeed important for correct ovarian function and fertility. We have observed that NKB (encoded by TAC3), NK3R (encoded by TACR3), and KISS1 (encoded by KISS1) expression is statistically significantly down-regulated in the cumulus cells of infertile patients
considered as a whole group, including with the most frequent disorders in women attending an IVF treatment: advanced maternal age, PCOS, endometriosis, LOR, and unexplained infertility. These results suggest that altered expression of these genes might be responsible, at least in part, for the infertility experienced by the patients.
In previous studies, we found that NKB/NK3R andvKISS1/KISS1R systems are present in human MGCs and CCs (19, 23–25). Moreover, we compared the expression of these systems between oocyte donors and infertile women with PCOS, and we found statistically significant differences (23). TAC3, TACR3, and KISS1 mRNAs were down-regulated in MGCs and CCs from PCOS patients, which led us to wonder whether these results would repeat in patients with other infertility etiologies. In fact, in the present study we observed similar results in the infertile patients group, the one including all etiologies. This makes sense because oocyte quality is affected in all these etiologies. Different ovarian stimulation programs did not modify or were unable to induce a recovery of the expression of the
TAC3/TACR3 and KISS1/KISS1R systems to the levels observed in healthy donors. All in all, an expression analysis of the NKB and KISS1 systems in MGCs or CCs would thus be useful to assess the fertility status of a patient. In the case of PCOS, it is very probable that other genes are affected and their expression levels vary depending on how PCOS is man-
ifested in the patient because it is a very heterogeneous disease. New knowledge on the genetic alterations behind this syndrome could allow us to stablish a better, more specific genetic profile for PCOS.

Each cause of infertility displayed different anthropometric and hormone data, allowing a specific profile for each type of disease. Controlled ovarian stimulation for IVF induces multiple follicular growth, which causes a dramatic variation of ovarian steroids E2 and P4 in comparison with
the physiologic levels of a natural menstrual cycle. According to our results, the observed dysregulation of the NKB/NK3R and KISS1/KISS1R systems cannot be attributed to differences in the hormone state. Instead, it seems to be directly related to the infertility status (Table 1 and Supplemental Table 2).
Two fertility trends of the 21st century have become evident in the Western countries: women are having fewer children, and they are delaying births to a later age. Furthermore, women who choose to delay motherhood may encounter delays and/or disappointment due to decreased fecundity (36). Age-related infertility comprises several causes leading to infertility. On the one hand, there is a reduction in the number of oocytes (ovarian reserve) as age increases. On the other hand, oocyte quality is also impaired with advancing age due to a widely described correlation between female age and oocyte chromosomal abnormalities,
leading to a higher rate of miscarriage and genetic disorders in the fetus. This higher frequency of aneuploidies is due to alterations in the regulatory machinery responsible for assembly of the oocyte meiotic spindle.

Furthermore, aging is also associated with the appearance of other infertility-related disorders such as tubal disease, leiomyomas, and endometriosis (37). In this study, the group of women of advanced maternal age showed a statistically significant down-regulation in KISS1, TAC3, and TACR3 levels, suggesting that altered expression of these genes might be involved in the impaired oocyte quality and/or the decreased ovarian reserve associated with advancing age. In fact, previous studies performed in different animal models have already found an association between kisspeptin and follicle development and oocyte maturation (18, 20, 26, 38). Endometriosis is a chronic inflammatory disease that cause pain and infertility in women, with a prevalence of 0.8% to 6.0% in population-based studies (39–41) and 20% to 50% in subfertile women (42, 43). Endometriosis is characterized by the growing of endometrial-like tissue in ectopic locations such as the oviduct, ovary, or peritoneal cavity. The origin and pathogenesis of this disease remains unclear, and different theories have been postulated to explain this phenomenon. Some theories propose that endometrial implants come from uterine endometrium, and other theories propose that these implants arise from other tissues, involving a process of transformation (44). The abnormally implanted tissue responds cyclically to hormones, developing inflammatory responses. Consequently, patients may develop pelvic adhesions and experience pain and infertility (45). Endometriosis has been related to impaired oocyte quality, reduced fertility, and lower implantation rates after IVF, but the link between infertility and endometriosis is still poorly
understood (46). Genetic and epigenetic changes have been associated with endometriosis, which is considered a hereditary disease, and many cases have been attributed to hereditary factors (47).
A recent study has detected that KISS1 expression is statistically significantly higher in endometriosis lesions in comparison with eutopic glandular endometrium, suggesting a possible role of KISS1 in endometriosis pathogenesis (48).
However, a different study did not detect KISS1 expression in any sample from endometriosis patients (49), which could be due to methodological or design differences between the studies. In our case, we analyzed granulosa cells from endometriosis patients and found that they constituted a very heterogeneous group in relation to the expression of the TAC3/ TACR3 and KISS1/KISS1R systems; when considered as a whole, only the expression of KISS1R was altered. KISS1R mRNA levels were statistically significantly higher in CCs from endometriosis patients in comparison with healthy oocyte donors. Thus, the increased expression of KISS1R could be one of the multiple factors involved in the origin of endometriosis and related infertility. These data suggest that endometriosis is a very different entity in comparison with advanced maternal age and LOR as a cause of infertility. As mentioned earlier, there is still much to know about the origin and causes of this disease.
Further studies are needed to clarify the reasons behind the increase in KISS1R expression and investigate the potential relationship between KISS1/KISS1R and endometriosis. If confirmed, KISS1 and/or its receptor could serve as biomarkers for endometriosis diagnosis and detection.
Low ovarian response indicates a reduction in the number of oocytes retrieved after an ovarian puncture due to a diminished follicular response to COS (30, 50). The existence of LOR was unveiled thanks to the increasing acceptance and spreading of ART. Approximately 10% of women undergoing IVF treatment will show LOR to COS. However, this incidence can be higher in the infertile population, as many affected
women never undergo an ART treatment (51). Regarding the results of our study, lower expression levels of KISS1 and TACR3 were observed in the MGCs of women with LOR in comparison with healthy oocyte donors.
This altered expression profile suggests the possible involvement of these factors in the correct follicle recruitment and development in response to gonadotropin stimulation. These results are concordant with previous studies that have identified the involvement of KISS1/KISS1R system in the regulation of follicular development, oocyte maturation, ovulation,
and ovarian steroidogenesis (28). Our results suggest that, besides kisspeptin expression levels, correct expression levels of NKB might also be necessary for normal folliculogenesis. In relation to advanced-age patients and those with LOR, it is worth pointing to a previous study performed in mice that revealed that a defect in the KISS1/KISS1R system induced a state similar to premature ovarian failure. Mutant mice
showed a premature decline in ovulatory rate, progressive loss of oocytes and antral follicles, and reduced fertility. This is concordant with results of our study because both older patients and low responders showed decreased levels of KISS1 in comparison with healthy oocyte donors (20).
CONCLUSION
Our study has revealed a differential and altered regulation of the NKB/NK3R and KISS1/KISS1R systems in cumulus and granulosa cells from women with infertility of different etiologies, particularly in patients with advanced age, endometriosis, and LOR. We provide evidence that an abnormal expression of these systems at the ovarian level might be
involved in the decreased fertility of these patients expression of these systems at the ovarian level might be involved in the decreased fertility of these patients.
REFERENCES
1. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–26.
2. Agarwal A, Majzoub A, Parekh N, Henkel R. A schematic overview of the
current status of male infertility practice. World J Mens Health 2019;37:e41.
3. Johnson MH. Essential reproduction. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2018.
4. Healy DL, Trounson AO, Andersen AN. Female infertility: causes and treatment. Lancet 1994;343:1539–44.
5. Candenas L, Lecci A, Pinto FM, Patak E, Maggi CA, Pennefather JN. Tachykinins and tachykinin receptors: effects in the genitourinary tract. Life Sci 2005;76:835–62.
6. De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003;100:10972 6.
7. Lehman MN, Coolen LM, Steiner RA, Neal-Perry G, Wang L, Moenter SM,
et al. The 3rd World Conference on Kisspeptin, ‘‘Kisspeptin 2017: Brain
and Beyond’’: Unresolved questions, challenges and future directions for
the field. J Neuroendocrinol 2018;30:e12600.
8. Page NM, Woods RJ, Gardiner SM, Lomthaisong K, Gladwell RT, Butlin DJ,et al. Excessive placental secretion of neurokinin B during the third trimester causes pre-eclampsia. Nature 2000;405:797–800.
9. Pinto FM, Armesto CP, Magraner J, Trujillo M, Martin JD, Candenas ML. Tachykinin receptor and neutral endopeptidase gene expression in the rat uterus: characterization and regulation in response to ovarian steroid treatment. Endocrinology 1999;140:2526–32.
10. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr,
Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J
Med 2003;349:1614–27.
11. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al.TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism
reveal a key role for Neurokinin B in the central control of reproduction.
Nat Genet 2009;41:354–8.
12. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev2012;92:1235–316.
13. Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 2004;11:2045–81.
14. Hu G, Lin C, He M, Wong AO. Neurokinin B and reproductive functions:
‘‘KNDy neuron’’ model in mammals and the emerging story in fish. Gen
Comp Endocrinol 2014;208:94–108.
15. Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets 2006;7:963–74.
16. Clarke H, Dhillo WS, Jayasena CN. Comprehensive Review on Kisspeptin and Its Role in Reproductive Disorders. Endocrinol Metab (Seoul) 2015;30:124–41.
17. Skorupskaite K, George JT, Veldhuis JD, Anderson RA. Neurokinin B Regulates Gonadotropin Secretion, Ovarian Follicle Growth, and the Timing of Ovulation in Healthy Women. J Clin Endocrinol Metab 2018;103:95–104.
18. Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology 2006;147:4852–62.
19. Cejudo Roman A, Pinto FM, Dorta I, Almeida TA, Hernandez M, Illanes M,et al. Analysis of the expression of neurokinin B, kisspeptin, and their
cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril 2012;97:1213–9.
20. Gaytan F, Garcia-Galiano D, Dorfman MD, Manfredi-Lozano M,
Castellano JM, Dissen GA, et al. Kisspeptin receptor haplo-insufficiency
causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology 2014;155:3088–97.




